Articles
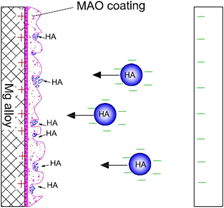
Characteristics of CeO2/ZrO2-HA composite coating on ZK60 magnesium alloy
-
- Published online by Cambridge University Press:
- 13 February 2017, pp. 1073-1082
-
- Article
- Export citation
JMR Early Career Scholars in Materials Science Annual Issue
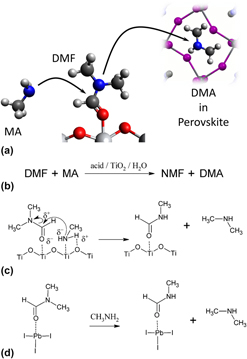
Transamidation of dimethylformamide during alkylammonium lead triiodide film formation for perovskite solar cells
-
- Published online by Cambridge University Press:
- 28 July 2016, pp. 45-55
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Articles
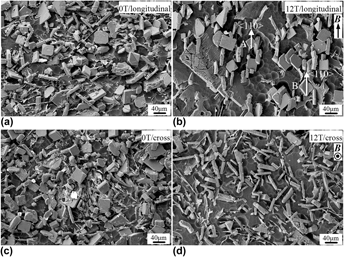
Influence of a high magnetic field on the solidification structures of ternary Al–Fe–Zr alloy
-
- Published online by Cambridge University Press:
- 06 February 2017, pp. 2035-2044
-
- Article
- Export citation
Crack initiation in the very high cycle fatigue regime of nitrided 42CrMo4 steel
-
- Published online by Cambridge University Press:
- 07 August 2017, pp. 4305-4316
-
- Article
- Export citation
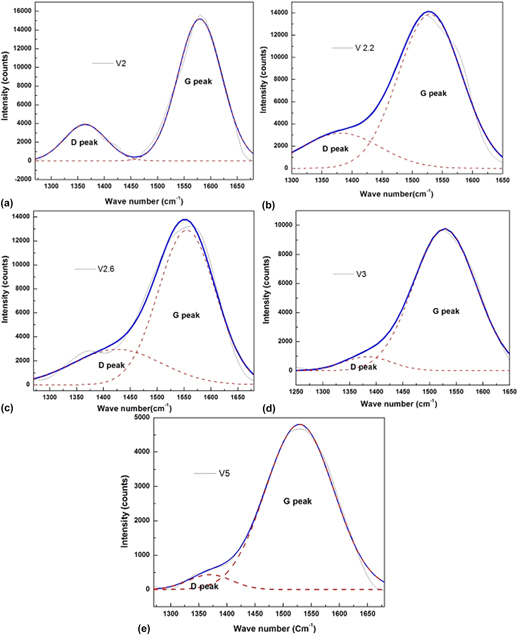
Ion beam energy dependence of surface and structural properties of amorphous carbon films deposited by IBSD method on Ni–Cu alloy
-
- Published online by Cambridge University Press:
- 14 February 2017, pp. 1258-1266
-
- Article
- Export citation
A modified constitutive model based on Arrhenius-type equation to predict the flow behavior of Fe–36%Ni Invar alloy
-
- Published online by Cambridge University Press:
- 03 July 2017, pp. 3831-3841
-
- Article
- Export citation
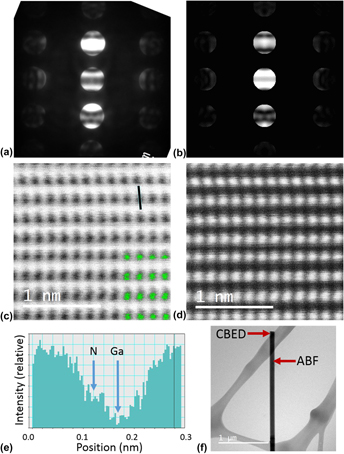
Comparison of convergent beam electron diffraction and annular bright field atomic imaging for GaN polarity determination
-
- Published online by Cambridge University Press:
- 13 December 2016, pp. 936-946
-
- Article
- Export citation
Transmission electron microscopy study of the microstructural evolution during high-temperature and low-stress (011) [11] shear creep deformation of the superalloy single crystal LEK 94
-
- Published online by Cambridge University Press:
- 15 September 2017, pp. 4491-4502
-
- Article
- Export citation
Invited Review
Strain landscapes and self-organization of free surfaces in complex oxide epitaxy
-
- Published online by Cambridge University Press:
- 22 August 2017, pp. 3958-3976
-
- Article
- Export citation
Articles
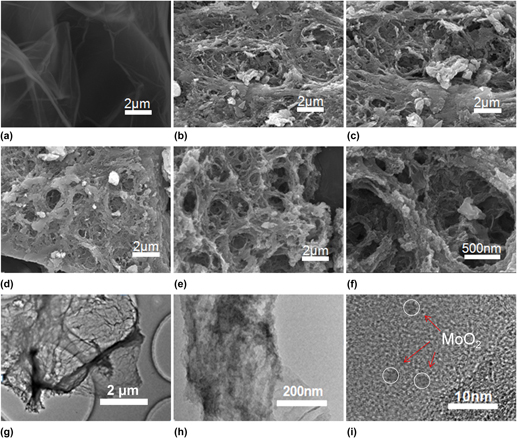
Enhanced supercapacitor performance based on 3D porous graphene with MoO2 nanoparticles
-
- Published online by Cambridge University Press:
- 28 November 2016, pp. 292-300
-
- Article
- Export citation
Invited Papers
Tailoring plasticity of metallic glasses via interfaces in Cu/amorphous CuNb laminates
-
- Published online by Cambridge University Press:
- 13 July 2017, pp. 2680-2689
-
- Article
- Export citation
Articles
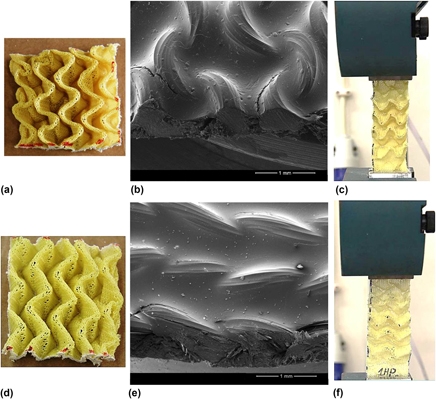
High-performance composite with negative Poisson’s ratio
-
- Published online by Cambridge University Press:
- 11 September 2017, pp. 3477-3484
-
- Article
- Export citation
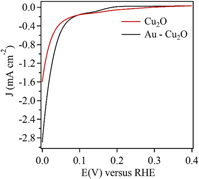
Effect of gold underlayer on copper(I) oxide photocathode performance
-
- Published online by Cambridge University Press:
- 18 April 2017, pp. 1656-1664
-
- Article
- Export citation
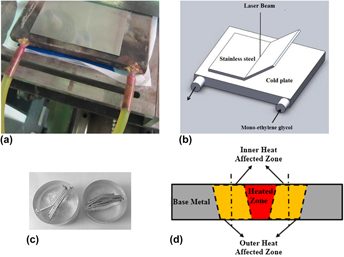
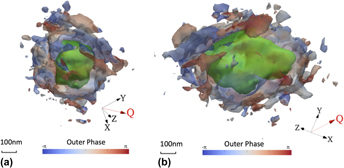
Deformation, microstructure, hardness, and pitting corrosion of 316 stainless steel after laser forming: A comparison between natural and forced cooling
-
- Published online by Cambridge University Press:
- 02 May 2017, pp. 3046-3054
-
- Article
- Export citation
Invited Paper
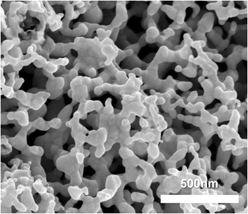
Direct solution-based reduction synthesis of Au, Pd, and Pt aerogels
-
- Published online by Cambridge University Press:
- 30 October 2017, pp. 4153-4165
-
- Article
- Export citation
Articles
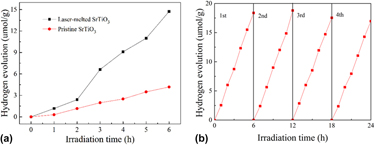
Defects enhanced photocatalytic performances in SrTiO3 using laser-melting treatment
-
- Published online by Cambridge University Press:
- 13 December 2016, pp. 748-756
-
- Article
- Export citation
Measuring optical properties of individual SnO2 nanowires via valence electron energy-loss spectroscopy
-
- Published online by Cambridge University Press:
- 15 May 2017, pp. 2479-2486
-
- Article
- Export citation
Drop-weight impact test on an integrated composite sandwich panel of aluminum honeycomb and epoxy resin
-
- Published online by Cambridge University Press:
- 22 May 2017, pp. 2258-2265
-
- Article
- Export citation
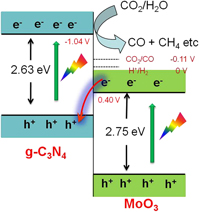
In situ preparation of Z-scheme MoO3/g-C3N4 composite with high performance in photocatalytic CO2 reduction and RhB degradation
-
- Published online by Cambridge University Press:
- 17 July 2017, pp. 3660-3668
-
- Article
- Export citation
Invited Reviews
Nucleation of fractal nanocrystallites upon annealing of Fe-based metallic glass
-
- Published online by Cambridge University Press:
- 13 March 2017, pp. 1880-1887
-
- Article
- Export citation