Volume 38 - December 1972
Research Article
The cause of coloration in Derbyshire Blue John banded fluorite and other blue banded fluorites
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 459-470
-
- Article
- Export citation
The thermal expansion behaviour of the framework silicates
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 593-604
-
- Article
- Export citation
Piemontite schists from Haast River, New Zealand
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 64-71
-
- Article
- Export citation
The Sitathali meteorite
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 335-343
-
- Article
- Export citation
A metallographic study of some hexahedrites
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 725-735
-
- Article
- Export citation
Melting relations of some lavas of Réunion Island, Indian Ocean
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 344-352
-
- Article
- Export citation
Evidence of shock-metamorphic effects in the Ergheo meteorite
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 197-204
-
- Article
- Export citation
Study of metamict Ceylon zircons
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 605-613
-
- Article
- Export citation
Calcium sulphosilicate in lime-kiln wall coating
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 968-971
-
- Article
- Export citation
The join CaMgSi2O6-Ca2MgSi2O7-CaTiAl2O6 in the system CaO-MgO-Al2O3-TiO2-SiO2 and its bearing on titanpyroxenes
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 471-480
-
- Article
- Export citation
X-Ray data and chemical analyses of some titanomagnetite and ilmenite samples from the Bushveld Complex, South Africa
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 863-871
-
- Article
- Export citation
A redetermination of the unit-cell geometry of scolecite
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 72-75
-
- Article
- Export citation
A microprobe study of chromites from the Andızlık-Zımparalık area, south-west Turkey
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 76-82
-
- Article
- Export citation
Further investigations into the nature of the materials chlorophaeite and palagonite
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 205-214
-
- Article
- Export citation
A precise and accurate method for the quantitative determination of carbonate minerals by X-Ray diffraction using a spiking technique
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 481-487
-
- Article
- Export citation
Preparation of glass standards for the use in X-ray microanalysis
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 614-617
-
- Article
- Export citation
A silica-deficient pyroxene in iron ore sinters
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 872-877
-
- Article
- Export citation
Short Communications
On the occurrence of Chlorapatite at the Emerald Mine, Rajagarh village, Ajmer
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 972-974
-
- Article
- Export citation
Research Article
A metallographie study of some iron meteorites of high nickel content
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 736-755
-
- Article
- Export citation
Effective molecular weights of the components in solution for the system anorthite-åermanite
-
- Published online by Cambridge University Press:
- 05 July 2018, pp. 353-357
-
- Article
- Export citation

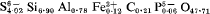 approximately 4[Ca
approximately 4[Ca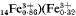 Al
Al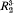 SiO
SiO