Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Pliego-Cuervo, Y.B.
and
Glasser, F.P.
1978.
Role of sulphates in cement clinkering: The calcium silicosulphate phase.
Cement and Concrete Research,
Vol. 8,
Issue. 4,
p.
455.
Irran, E.
Tillmanns, E.
and
Hentschel, G.
1997.
Ternesite, Ca5(SiO4)2SO4, a new mineral from the Ettringer Bellerberg/Eifel, Germany.
Mineralogy and Petrology,
Vol. 60,
Issue. 1-2,
p.
121.
Cheng, Jun
Zhou, Junhu
Liu, Jianzhong
Zhou, Zhijun
Huang, Zhenyu
Cao, Xinyu
Zhao, Xiang
and
Cen, Kefa
2003.
Sulfur removal at high temperature during coal combustion in furnaces: a review.
Progress in Energy and Combustion Science,
Vol. 29,
Issue. 5,
p.
381.
Sherman, Steven R.
2009.
Nuclear powered CO2 capture from the atmosphere.
Environmental Progress & Sustainable Energy,
Vol. 28,
Issue. 1,
p.
52.
Hanein, Theodore
Galan, Isabel
Glasser, Fredrik P.
Skalamprinos, Solon
Elhoweris, Ammar
Imbabi, Mohammed S.
and
Bannerman, Marcus N.
2017.
Stability of ternesite and the production at scale of ternesite-based clinkers.
Cement and Concrete Research,
Vol. 98,
Issue. ,
p.
91.
Blanco-Varela, M.T.
Carmona-Quiroga, P.M.
Diouri, A.
Boukhari, A.
Ait Brahim, L.
Bahi, L.
Khachani, N.
Saadi, M.
Aride, J.
and
Nounah, A.
2018.
Ternesite as a component of sulfobelitic cements.
MATEC Web of Conferences,
Vol. 149,
Issue. ,
p.
01011.
Skalamprinos, Solon
Jen, Gabriel
Galan, Isabel
Whittaker, Mark
Elhoweris, Ammar
and
Glasser, Fredrik
2018.
The synthesis and hydration of ternesite, Ca5(SiO4)2SO4.
Cement and Concrete Research,
Vol. 113,
Issue. ,
p.
27.
Blanco-Varela, M.T.
Carmona-Quiroga, P.M.
Diouri, A.
Boukhari, A.
Ait Brahim, L.
Bahi, L.
Khachani, N.
Saadi, M.
Aride, J.
and
Nounah, A.
2018.
Ternesite as a component of sulfobelitic cements.
MATEC Web of Conferences,
Vol. 149,
Issue. ,
p.
01011.
Böhme, Nadine
Hauke, Kerstin
Neuroth, Manuela
and
Geisler, Thorsten
2020.
In Situ Hyperspectral Raman Imaging of Ternesite Formation and Decomposition at High Temperatures.
Minerals,
Vol. 10,
Issue. 3,
p.
287.
Dvořák, Karel
Gazdič, Dominik
and
Fridrichová, Marcela
2020.
The influence of firing parameters on the crystallinity of ternesite.
Journal of Crystal Growth,
Vol. 542,
Issue. ,
p.
125691.
Jing, Guojian
Zhang, Jinpu
Lu, Xiaolei
Xu, Jiankai
Gao, Yang
Wang, Shuxian
Cheng, Xin
and
Ye, Zhengmao
2020.
Comprehensive evaluation of formation kinetics in preparation of ternesite from different polymorphs of Ca2SiO4.
Journal of Solid State Chemistry,
Vol. 292,
Issue. ,
p.
121725.
Bullerjahn, F.
Scholten, T.
Scrivener, K.L.
Ben Haha, M.
and
Wolter, A.
2020.
Formation, composition and stability of ye'elimite and iron-bearing solid solutions.
Cement and Concrete Research,
Vol. 131,
Issue. ,
p.
106009.
Liu, Lei
Zhang, Wensheng
Ren, Xuehong
Ye, Jiayuan
Zhang, Jiangtao
and
Qian, Jueshi
2021.
Formation, structure, and thermal stability evolution of ternesite based on a single-stage sintering process.
Cement and Concrete Research,
Vol. 147,
Issue. ,
p.
106519.
Shen, Yan
Wang, Peifang
Chen, Xi
Zhang, Wei
and
Qian, Jueshi
2021.
Synthesis, characterisation and hydration of ternesite.
Construction and Building Materials,
Vol. 270,
Issue. ,
p.
121392.
Liu, Lei
Zhang, Wensheng
Ren, Xuehong
Ye, Jiayuan
Zhang, Jiangtao
Luo, Zhongtao
and
Qian, Jueshi
2022.
Sintering Behaviour and Structure-Thermal Stability Relationships of Alkali-Doped Ternesite.
SSRN Electronic Journal ,
Huang, Yongbo
Dong, Dong
Wang, Xuecheng
Zhang, Zixu
Zhao, Piqi
Cui, Na
and
Lu, Lingchao
2022.
Ternesite-calcium sulfoaluminate cement: Preparation and hydration.
Construction and Building Materials,
Vol. 344,
Issue. ,
p.
128187.
Shen, Yan
Chen, Xi
Li, Jiang
Wang, Peifang
and
Qian, Jueshi
2022.
Preparation and Performance of Ternesite–Ye’elimite Cement.
Materials,
Vol. 15,
Issue. 12,
p.
4369.
Huang, Xiaofei
Shi, Fei
Wang, Guoling
Yu, Jiangbo
Ma, Suhua
and
Li, Weifeng
2022.
Kinetics and Mechanism of Ternesite Formation from Dicalcium Silicate and Calcium Sulfate Dihydrate.
Materials,
Vol. 15,
Issue. 7,
p.
2626.
Liu, Lei
Zhang, Wensheng
Ren, Xuehong
Ye, Jiayuan
Zhang, Jiangtao
Luo, Zhongtao
and
Qian, Jueshi
2023.
Sintering behaviour and structure-thermal stability relationships of alkali-doped ternesite.
Cement and Concrete Research,
Vol. 164,
Issue. ,
p.
107043.
Song, Fengyu
Huo, Didi
Wang, Yanmin
Su, Dunlei
and
Liu, Xiao-Cun
2024.
Effect of phosphorus-doping on ternesite: Structure, thermal stability and hydration.
Construction and Building Materials,
Vol. 432,
Issue. ,
p.
136650.
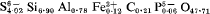 approximately 4[Ca5(SiO4)2SO4] and isostructural with silicocarnotite, 4[Ca5(PO4)2SiO4]. Type material is preserved at the Government Chemical Laboratories, Perth, Western Australia.
approximately 4[Ca5(SiO4)2SO4] and isostructural with silicocarnotite, 4[Ca5(PO4)2SiO4]. Type material is preserved at the Government Chemical Laboratories, Perth, Western Australia.