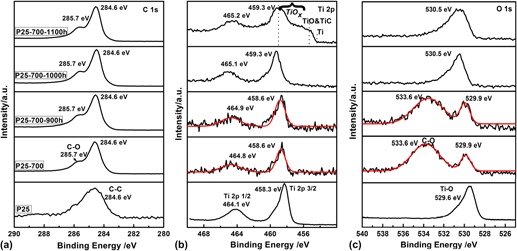
Focus Issue: Titanium Dioxide Nanomaterials
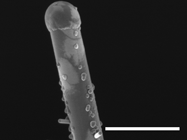
Scanning electron micrograph showing the microstructure of pure TiO2 nanotubes at room temperature. [I.M. Low, H. Albetran, V.M. Prida, V. Vega, P. Manurung, and M. Ionescu: A comparative study on crystallization behavior, phase stability and binding energy in pure and Cr-doped TiO2 nanotubes. p. 304.]
Articles
Enhanced photocatalytic activity of TiO2–niobate nanosheet composites
-
- Published online by Cambridge University Press:
- 19 November 2012, pp. 424-430
-
- Article
- Export citation
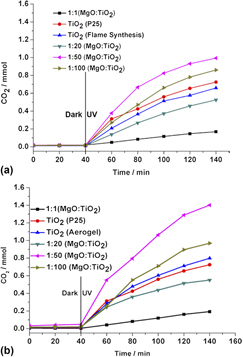
MgO–TiO2 mixed oxide nanoparticles: Comparison of flame synthesis versus aerogel method; characterization, and photocatalytic activities
-
- Published online by Cambridge University Press:
- 19 September 2012, pp. 431-439
-
- Article
- Export citation
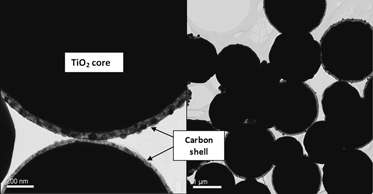
Synthesis and study of carbon/TiO2 and carbon/TiO2 core–shell micro-/nanospheres with increased density
-
- Published online by Cambridge University Press:
- 29 October 2012, pp. 440-448
-
- Article
- Export citation
Synthesis of TiO2@C core–shell nanostructures with various crystal structures by hydrothermal and postheat treatments
-
- Published online by Cambridge University Press:
- 29 August 2012, pp. 449-453
-
- Article
- Export citation
Carbothermal synthesis of titanium oxycarbide as electrocatalyst support with high oxygen evolution reaction activity
-
- Published online by Cambridge University Press:
- 09 November 2012, pp. 454-460
-
- Article
- Export citation
Processing and functionalization of conductive substoichiometric TiO2 catalyst supports for PEM fuel cell applications
-
- Published online by Cambridge University Press:
- 17 October 2012, pp. 461-467
-
- Article
- Export citation
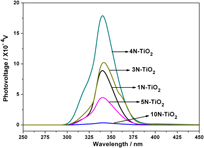
Preparation and photovoltaic properties of N-doped TiO2 nanocrystals in vacuum
-
- Published online by Cambridge University Press:
- 08 January 2013, pp. 468-474
-
- Article
- Export citation
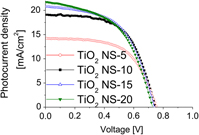
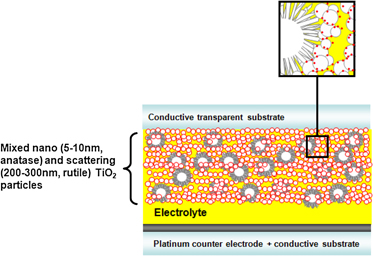
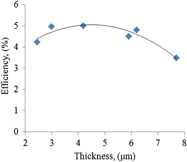
The effect of photoanode thickness on the performance of dye-sensitized solar cells containing TiO2 nanosheets with exposed reactive {001} facets
-
- Published online by Cambridge University Press:
- 28 December 2012, pp. 475-479
-
- Article
- Export citation
Thin single screen-printed bifunctional titania layer photoanodes for high performing DSSCs via a novel hybrid paste formulation and process
-
- Published online by Cambridge University Press:
- 23 November 2012, pp. 480-487
-
- Article
- Export citation
A study of TiO2 binder-free paste prepared for low temperature dye-sensitized solar cells
-
- Published online by Cambridge University Press:
- 21 November 2012, pp. 488-496
-
- Article
- Export citation
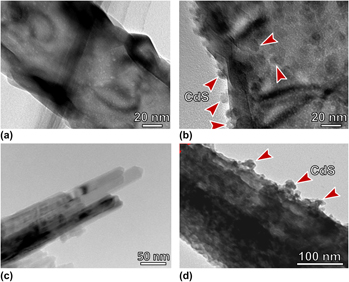
CdS-sensitized TiO2 photoelectrodes for quantum dots-based solar cells
-
- Published online by Cambridge University Press:
- 08 January 2013, pp. 497-501
-
- Article
- Export citation
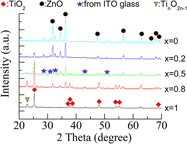
Ink-jet-printed (ZnO)1−x(TiO2)x composite films for solar cell applications
-
- Published online by Cambridge University Press:
- 15 October 2012, pp. 502-506
-
- Article
- Export citation
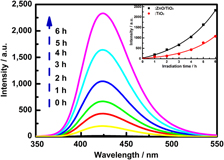
A novel route to ZnO/TiO2 heterojunction composite fibers
-
- Published online by Cambridge University Press:
- 29 August 2012, pp. 507-512
-
- Article
- Export citation
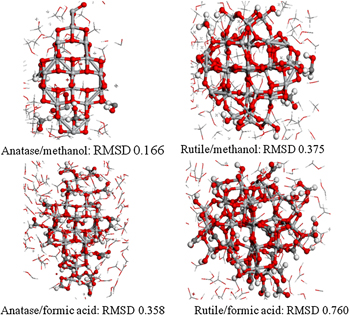
Molecular dynamics simulations of the interactions between TiO2 nanoparticles and water with Na+ and Cl−, methanol, and formic acid using a reactive force field
-
- Published online by Cambridge University Press:
- 29 November 2012, pp. 513-520
-
- Article
- Export citation
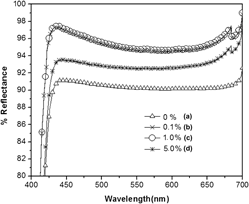
Study on reflectivity and photostability of Al-doped TiO2 nanoparticles and their reflectors
-
- Published online by Cambridge University Press:
- 27 November 2012, pp. 521-528
-
- Article
- Export citation
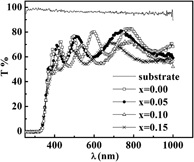
Magnetic, structural, electronic, and optical investigations of Ti1−xMnxO2 films
-
- Published online by Cambridge University Press:
- 31 July 2012, pp. 529-534
-
- Article
- Export citation
Indium tin oxide modified by titanium dioxide nanoparticles dispersed in poly(N-vinylpyrrolidone) for use as an electron-collecting layer in organic solar cells with an inverted structure
-
- Published online by Cambridge University Press:
- 29 August 2012, pp. 535-540
-
- Article
- Export citation
Front Cover (OFC, IFC) and matter
JMR volume 28 issue 3 Cover and Front matter
-
- Published online by Cambridge University Press:
- 01 February 2013, pp. f1-f6
-
- Article
-
- You have access
- Export citation
Back Cover (OBC, IBC) and matter
JMR volume 28 issue 3 Cover and Back matter
-
- Published online by Cambridge University Press:
- 01 February 2013, pp. b1-b2
-
- Article
-
- You have access
- Export citation