Vitamin A (VA) plays an important role during pregnancy as a regulator of embryonic development, including the epidermis( Reference Mammadova, Zhou and Carels 1 ). This occurs through retinoic acid, an active derivate of VA that synthesises and degrades enzymes alongside participating in spermatogenesis and embryogenesis( Reference Mammadova, Zhou and Carels 1 , Reference Clagett-Dame and Knutson 2 ). VA status during pregnancy has been associated with maternal–child health outcomes, such as birth weight, lung function, bone mineralisation and increasing natural antibody concentrations in offspring, as shown in various studies worldwide( Reference Cohen, Kahn and Platt 3 – Reference Palmer, Schulze and Khatry 6 ).
Varied food sources provide VA to the human beings, such as viscera, dairy products, eggs (preformed VA esters, such as retinoid) and orange–yellow and dark-green vegetables (VA precursors, mainly β-carotene)( 7 ). The bioavailability of VA precursors in the organism is less efficient than for preformed VA esters, thus affecting the VA functions depending on the food matrices from which the vitamin was obtained( 7 , Reference Van Loo-Bouwman, Naber and Schaafsma 8 ). Hepatic storage is the main site where VA can be found in the humans, and varied methods were developed attempting to measure retinol stores in the organ, serum retinol (µmol/l) being the widest used and recommended method in epidemiological surveys( 7 ).
VA deficiency is still an important public health problem in low- and middle-income countries. The global prevalence of VA deficiency during pregnancy is estimated to be 15·3 %, considering serum retinol concentrations <0·7 µmol/l, and 7·8 % based on reported frequency of night blindness( 7 ). The latest global estimates for pregnant women in Latin America and the Caribbean showed a prevalence of 2 % for low serum retinol and 4·4 % for night blindness; however, some estimates were obtained through regression-based models due to the lack of available data for most countries in the region( 7 ). In Brazil, a national representative study with childbearing-age women (15–49 years) found a prevalence of 12·3 % for VA deficiency and an alarming prevalence of 49·2 % (serum retinol <1·05 µmol/l) for VA insufficiency( 9 ). Thus, the identification of the predictors for VA status during pregnancy may help in the prevention of inadequate VA nutritional status, targeting safe interventions for improvement of VA status during this period. Considering that supplementation during pregnancy might be harmful to the mother–child pair owing to teratogenic effects if higher than 10 000 IU (3·0 mg) daily or 25 000 IU (7·5 mg) weekly( 10 ), other strategies to prevent VA insufficiency or deficiency must be considered.
Poor VA status in pregnancy is caused mainly due to inadequacies in dietary intake of VA-rich foods as well as during infectious episodes( 7 ). Alternatively, Zn and Fe deficiencies have been reported to occur concomitantly with VA deficiency due to metabolic pathways shared by these micronutrients( Reference Thorne-Lyman and Fawzi 11 , Reference Tanumihardjo, Russell and Stephensen 12 ). A national analysis in Brazil compared nutrient intake among pregnant women through two non-consecutive food records, showing elevated inadequacies for Fe (97 %), VA (71 %) and Zn (35 %) according to estimated average requirements( Reference dos Santos, Sichieri and Marchioni 13 ).
There is a lack of studies on the predictors of VA status at the beginning of the third trimester of pregnancy, a crucial moment when the maternal VA status has a positive correlation with the baby’s liver stores in the first 6 months after birth( Reference Ramalho and Dolinsky 14 ). Likewise, there is no prospective study in Northern Brazil regarding the nutritional status of micronutrients in pregnant women. Therefore, our aim was to investigate the predictors of VA status at the beginning of the third trimester of pregnancy among women living in the Western Brazilian Amazon.
Methods
Study setting and population
This is a prospective cohort study conducted in the urban area of Cruzeiro do Sul, Acre State, Western Brazilian Amazon (latitude: 07° 37′ 52″ S; longitude: 72° 40′ 12″ W), part of the ‘MINA-Brazil – Maternal and Child Health and Nutrition in Acre, Brazil’ Birth Cohort study. Cruzeiro do Sul is the second largest city in Acre, with roughly 80 000 inhabitants, half of them women. The 2010 municipal-level Human Development Index for Cruzeiro do Sul was classified as medium (0·664). It is nearly 640 km from the Acre’s capital city, Rio Branco. Based on the 2010 demographic census, only 12·7 % of the households had access to proper sanitation in Cruzeiro do Sul( 15 ).
Our sample was composed of pregnant women enrolled in antenatal care in all primary health units (n 13) of the family health strategy in the urban area of the municipality. Women up to 20 weeks of pregnancy as measured by the last menstrual period (LMP), who were living in the city and intended to deliver at the only maternity hospital in Cruzeiro do Sul, were considered eligible for this study. It was estimated to track approximately 854 pregnant women, taking into account the number of deliveries at the local maternity hospital in 2013 (n 1780) as well as a proportion of 60 % of these women living in the urban area and a coverage of 80 % for the local primary healthcare.
Screening and recruitment of participants took place on a weekly basis by the nurses of each primary health unit, from February 2015 to January 2016. During this period, all eligible women were invited to participate in our study through phone calls or home visits. Afterwards, a home visit was scheduled to obtain written consent and to perform the socio-economic and health interview. Twin pregnancies were excluded from this analysis.
Cohort procedures, exposure and outcome variables
Trained field workers conducted face-to-face socio-economic and health interviews with pregnant women up to 20 weeks of pregnancy. We gathered information on ‘socio-demographic variables’ (maternal age, maternal skin colour (white or non-white), maternal schooling (≤9 or >9 years), head of the family (pregnant woman or other), living with a partner (no or yes), beneficiary of Bolsa Família conditional cash transfer programme (no or yes) and maternal occupation (unpaid job or paid job)); ‘environmental characteristics’ (water supply (general water distribution or water well/natural source), sewage collection (septic tank or open air/river), number of people in the household, type of household (masonry or wood/mix) and having a smoker in the house (no or yes)) and ‘clinical and obstetric history’ (menarche age, history of malaria (no or yes), history of abortion/stillbirth (no or yes) and number of live births).
Subsequently, between March 2015 and March 2016, all pregnant women were scheduled for the first evaluation between 16 and 20 weeks of pregnancy based on LMP. During the evaluation, the following data were gathered: anthropometric measures (weight, height and pre-pregnancy weight), food frequency consumption, current vaginal infections (no or yes), lifestyle behaviours during pregnancy (smoking: no or yes; alcohol use: no or yes), supplement use (none, folic acid+Fe and multiple micronutrients with VA), fasting venous blood samples and season at blood drawn (Amazonian summer season from May to October or Amazonian winter season from November to April) and gestational age (GA). Trained physicians performed obstetric ultrasound assessments to set the GA precisely, which was then compared with GA based on LMP( Reference Pincelli, Neves and Lourenço 16 ). The best GA estimate was obtained based on the following criteria: the LMP was used when participants reported regular menstrual cycles, no use of hormonal contraceptive methods, and when agreement between LMP and ultrasound estimates of GA was ≤7 d for pregnancies <20 weeks or ≤14 d for pregnancies from 20 to 28 weeks; ultrasound measurements were used in all other cases.
A second evaluation was held at the beginning of the third trimester of pregnancy between May 2015 and May 2016, according to the best GA estimate. All the data described for the first evaluation were collected again. For the present analysis, serum retinol at the second evaluation was considered as the outcome variable.
Laboratory procedures and analyses
Nurse technicians collected a fasting (8 h) venous blood sample (approximately 10 ml) on the morning of the scheduled day in both evaluations. Blood Hb was determined at the time of blood collection by a portable haemoglobinometer (Hemocue® Hb301; Hemocue)( Reference Burger and Pierre-Louis 17 ). The serum samples were collected in a dry test tube, protected from light and centrifuged within 2 h of collection. Serum was frozen at –20°C before it was sent to the Laboratory of Human Nutrition (School of Public Health, University of São Paulo) on dry ice and maintained at –70°C until analysis (within 6 months of blood drawn). Serum concentrations of retinol, β-carotene and 25-hydroxy vitamin D3 were measured using HPLC methods (HP-1100 HPLC system; Hewlett Packard)( Reference Gomes, Alves and Sevanian 18 ). At the second evaluation, C-reactive protein (CRP) was measured as a marker of acute inflammation, using an IMMAGE Immunochemistry System (Beckman Coulter). Biochemical data were double-typed using Excel for Windows and then converted to Stata 14.0 for data processing and analysis. Intra- and inter-assay CV were <7 %.
Anthropometric assessment
Weight and height were measured by trained researchers according to standardised procedures( 19 , Reference Lohman, Roche and Martorell 20 ). Body weight was measured with a Tanita Corporation portable scale, model UM061, with a capacity of 150 kg and a variation of 0·1 kg. Pregnant women were barefoot and wearing light clothes, standing erect with their feet together and arms extended along the body and maintained the position until reading and recording was completed. To measure height, a portable stadiometer (Alturaexata®) with precision of 0·1 mm and an extension of 213 cm was used. Pregnant women were barefoot, with their head free of accessories and hairstyles (ponytail, braid), positioned in the centre of the equipment, erect with arms extended along the body with the head kept high and looking at a fixed point at eye level. Heels, shoulders and buttocks of the pregnant women were pressed against the stadiometer, and the feet formed a right angle with the legs. Each anthropometric measurement was taken in duplicate, and mean values were used for calculation of BMI. The weekly gestational weight gain was calculated using the difference between the participant’s body weight at the second evaluation and that of the first evaluation, divided by the GA in weeks in the interval between the two. The weekly gestational weight gain was classified as insufficient, adequate and excessive according to guidelines from the Institute of Medicine based on the pre-pregnancy BMI( 21 ).
Food consumption assessment
A FFQ previously used for screening of micronutrient deficiencies among Amazonian scholars was adapted for this study( Reference Augusto, Cobayashi and Cardoso 22 ). The frequency of food group consumption (dairy products, beans, green and root vegetables, fruits, Amazonian fruits, eggs, meat, viscera, fish, cereals and breads, oils and ultra-processed) was estimated during the last month, with the following response options: never, 1–3 times/month, 1–3 times/week, 4–6 times/week, 1 time/d, 2–3 times/d and ≥4 times/d( Reference Augusto, Cobayashi and Cardoso 22 ). For this analysis, we considered some VA-rich foods grouped as follows: fruits and vegetables (no/monthly, weekly or daily consumption), Amazonian fruits (e.g. açaí – Euterpe oleracea Mart.; buriti – Maurita flexuosa L.f.; cajá – Spondias mombin L.; star fruit – Averrhoa carambola L.; cupuaçu – Theobroma grandiflorum (Willd. ex Spreng.) K.Schum; graviola – Annona muricata L.; jaca – Artocarpus heterophyllus Lam.; jambo – Syzygium malaccense (L.) Merr. & L.M. Perry; pupunha – Bactris gasipaes Kunth; tucumã – Astrocaryum aculeatum G.Mey. – no/rare or weekly consumption), viscera (e.g. liver, gizzard, heart – no/rare, monthly or weekly consumption), fish (no/monthly, weekly or daily consumption), dairy products (no/monthly, weekly, daily or ≥1 times/d consumption) and eggs (no/monthly, weekly or daily consumption). To set this group of VA food matrices in the diet, we used the Brazilian Food Composition Table( 23 ) and the publication ‘Brazilian Regional Foods’ from the Ministry of Health of Brazil( 24 ).
Ethical concerns
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all research procedures were approved by the Human Ethical Review Board of the School of Public Health, University of São Paulo (number 872.613, 13 November 2014). Written informed consent was obtained from each participant. For teenage pregnancies, the adolescent’s caregiver gave consent.
Power calculation
The sample size estimation was based on detecting predictors of serum retinol in the third trimester of pregnancy (at least 10 % of variation). For a power of 95 % with a two-tailed level of significance of 5 %, at least 120 participants were required.
Data analysis
The normality of continuous variables was first analysed with the Shapiro–Wilk test. Thus, a square-root transformation was used for the serum retinol at the second evaluation. The following data were used as continuous variables: age, number of people in the household, menarche age, number of live births, GA at first evaluation, pre-pregnancy BMI and all biochemical indicators. We compared characteristics of participants between evaluations using test of proportions, t test and Wilcoxon signed-rank test.
For this study, exposures measured at the first evaluation (16–20 weeks of pregnancy) were used in multiple linear regression models to identify predictors of serum retinol at the second evaluation (approximately 28 weeks of pregnancy). The selection of independent variables followed a hierarchical model with five levels of determination( Reference Victora, Huttly and Fuchs 25 ) ordered according to the influence on the outcome: (i) ‘sociodemographic and economic’: maternal age, maternal schooling, maternal skin colour, head of the family, living with a partner, beneficiary of Bolsa Família cash transfer programme and maternal occupation; (ii) ‘environmental’: water supply, sewage collection, number of people in the household, type of household and having a smoker in the house; (iii) ‘clinical and obstetric history’: menarche age, history of malaria, history of abortion/stillbirth and number of live births; (iv) ‘antenatal care’: pre-pregnancy BMI, current vaginal infections, smoking during pregnancy, alcohol use during pregnancy, seasonality of blood drawn, food consumption for each item and maternal supplementation and (v) ‘biochemical’: retinol, Hb, β-carotene and vitamin D at the first evaluation. We assumed GA and CRP concentrations at the second evaluation as controlling variables for the analysis in levels (iv) and (v). At each level of determination, independent variables were retained in the model if they were associated with the outcome at P<0·10 or if their inclusion in the model changed the R 2 by 10 % or more. Missing data were included in the multiple models by creating missing value categories. All analyses were performed using Stata 14.0 (Stata Corp.).
Results
The flowchart of participants is presented in Fig. 1. Of the 860 women screened for participation, 699 met eligibility requirements. After losses to missed appointments, miscarriage/abortion, refusal to participate, insufficient blood samples and twin pregnancies, 442 women had complete data and were included in the analysis. There was no significant difference in general characteristics among participants lost to follow-up and pregnant women included in the analyses (P>0·10).
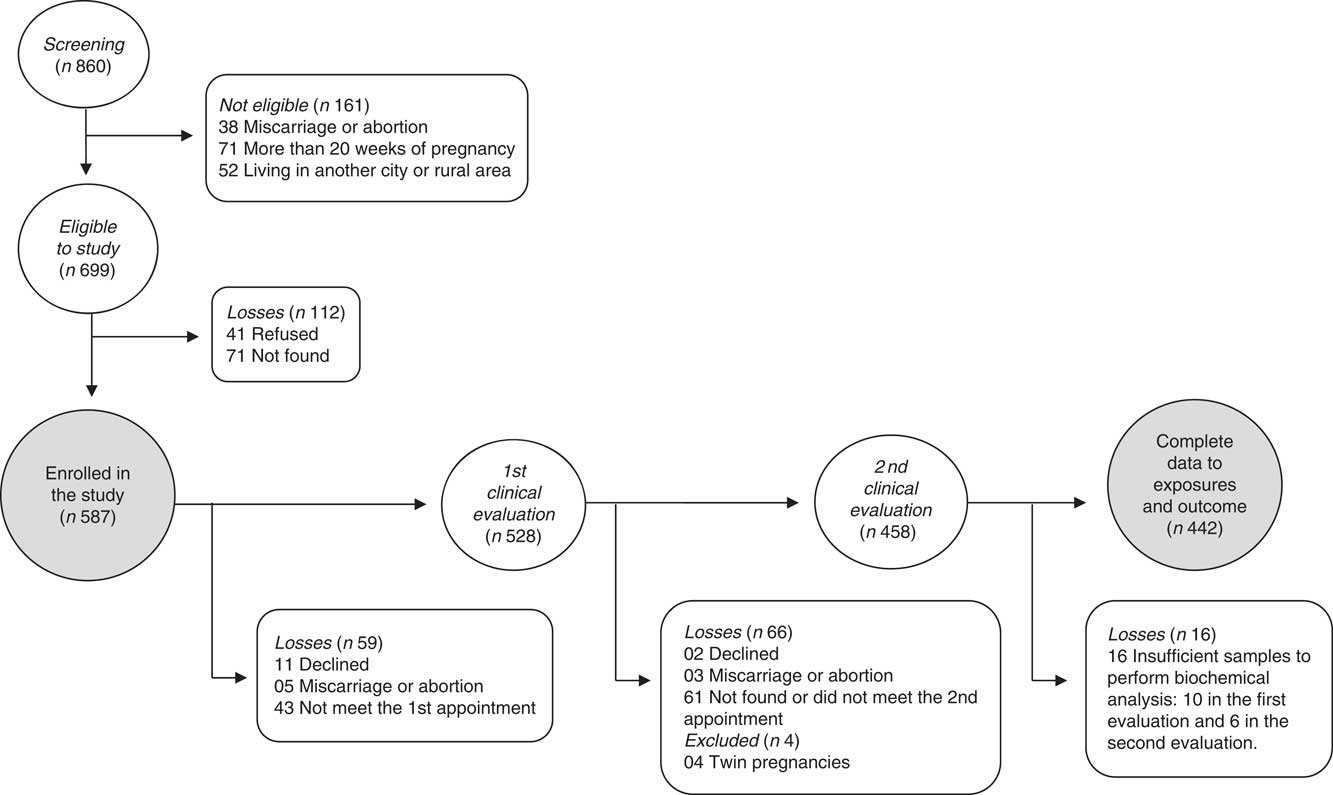
Fig. 1 Flowchart of the recruitment and follow-up of the pregnant women participants in the MINA-Brazil prospective study.
General characteristics of the participants are shown in Table 1. More than one quarter of the participants were teenagers (<19 years, 27·4 %). Most of the women had more than 9 years of schooling (67·58 %), were not the head of the family (86·28 %), were non-white (85·76 %), were living with a partner (77·36 %), had general water supply (62·95 %) and used a septic tank for sewage (67·36 %). The mean number of people living in the same household was 4·11 (sd 2·11), and 70·55 % of the participants lived in households with no smokers. Regarding overall antenatal care information, most of the participants experienced excessive weight gain between the two evaluations (59·17 %). More than half of the pregnant women reported at least one episode of malaria throughout their life (data not shown).
Table 1 Characteristics of the pregnant women from the MINA-Brazil prospective studyFootnote * (Numbers and percentages; mean values and standard deviations)

* Totals differ due to missing values.
† Only for those who have been previously pregnant.
‡ According to the Institute of Medicine guidelines, 2013.
Table 2 shows the comparisons of study participants between the two evaluations. Differences in mean values between evaluations were seen in the biochemical indicators, with an improvement of nutritional status for retinol, β-carotene and vitamin D at the second evaluation compared with the first one. Conversely, a reduction could be observed in mean serum Hb, followed by an increase in the frequency of anaemia. Online Supplementary Tables S1 and S2 present comparisons of the VA food sources consumption between the evaluations and by age-group at the first evaluation, respectively. There were significant differences for the consumption of viscera, fish and dairy products between evaluations and for fruits and vegetables, fish, dairy products and eggs between adolescents and adults.
Table 2 Characteristics of participants from the MINA-Brazil prospective study between the second and third trimester of pregnancy (Numbers and percentages; mean values and standard deviations; medians and interquartile ranges (IQR))
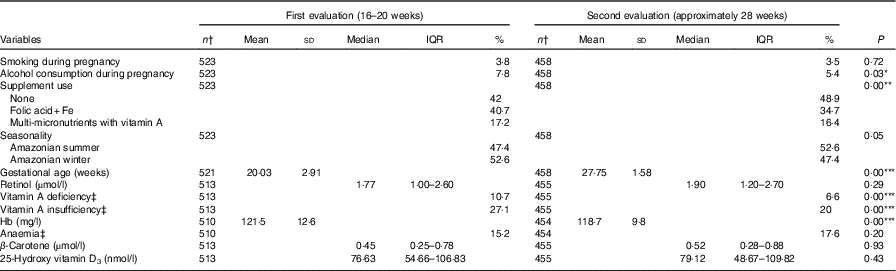
* P<0·05; ** P<0·01; *** P<0·001.
† Totals differ due to missing values.
‡ Vitamin A deficiency: serum retinol <0·7 µmol/l; vitamin A insufficiency: serum retinol <1·05 µmol/l; anaemia: Hb <110 mg/l.
In the final multiple regression model, variables positively associated with the serum retinol in the last trimester of pregnancy were seasonality, weekly consumption of Amazonian fruits and serum retinol at the first evaluation. The winter season (rainy season) was responsible for increasing serum retinol by 0·134 µmol/l compared with the summer season (dry season). The mean retinol concentrations at the beginning of the third trimester was 0·087 µmol/l higher among women who consumed Amazonian fruits weekly than among those who consumed these fruits less often. Conversely, having a smoker in the house presented a negative correlation, with a mean serum retinol of 0·087 µmol/l lower when compared with pregnant women without a smoker in the house. These four variables were responsible for explaining 11·1 % of variability in serum retinol (Table 3). Control for CRP concentrations had a non-significant effect on the R 2 and thus lowering it. Hence, we did not include this variable in the final model. Stratified analyses for predictors of serum retinol in the last trimester of pregnancy by adolescents and adults are presented in online Supplementary Tables S3 and S4, respectively. Among pregnant adolescents, paid maternal occupation was positively associated with serum retinol, while the low consumption of dairy products (no/monthly consumption) was negatively associated with the outcome when compared with pregnant adolescents who reported consumption of dairy products more than once a day. The consumption of fish and eggs was not associated with the serum retinol at mid-pregnancy neither in the first unadjusted analysis nor in the stratified analysis (data not shown in tables).
Table 3 Predictors of serum retinol at the beginning of the third trimester of pregnancy in the MINA-Brazil prospective study (β-Coefficients and 95 % confidence intervals; n 442)
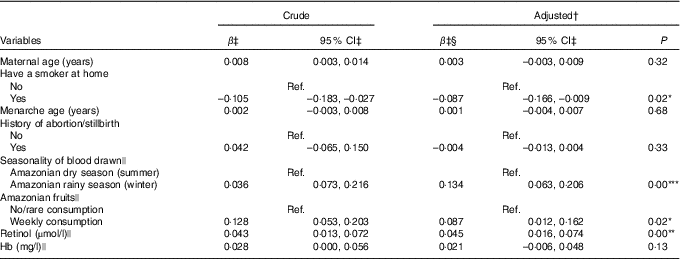
* P<0·05, ** P<0·01, *** P<0·001.
† R 2-adjusted: 11·1 %.
‡ Values are in the square root-transformed scale.
§ Controlled for gestational age at the second evaluation.
|| Measured at the first evaluation.
Discussion
In this prospective cohort study in the Western Brazilian Amazon, we showed that positive predictors of serum retinol at the beginning of the third trimester of pregnancy were seasonality (Amazon winter season), weekly consumption of Amazonian carotenoid-rich fruits and serum retinol in the second trimester. In contrast, having a smoker in the household reduced retinol concentrations. Our final model explained 11·1 % of the variability of serum retinol in the last trimester of pregnancy. Analyses by age-group showed few differences in adult pregnant women compared with the first set of predictors, as the association with the weekly consumption of Amazonian carotenoid-rich fruits was smoothed. On the other hand, we have seen different predictors of serum retinol at mid-pregnancy among adolescents, mainly socio-economic factors such as being enrolled in a paid occupation, were positively associated with serum retinol. Low consumption of dairy products was negatively associated with serum retinol in pregnant adolescents in this study. To our knowledge, this is the first study to measure the serum VA concentrations in two different periods of pregnancy. It is also the first prospective investigation of predictors of serum retinol during pregnancy in a low-income area in Brazil.
Literature on the determinants of serum retinol in mid-gestation is scarce; most available data analysed either the determinants of retinol in the final gestational weeks or VA deficiency during pregnancy or postpartum( Reference Kæstel, Martinussen and Aaby 26 , Reference Van Stuijvenberg, Schoeman and Nel 27 ). This study is the first to address this subject in this specific period in a less-developed area of Brazil, which is relevant in view of the physiological characteristics of the forthcoming gestational weeks. Throughout the third trimester, fetus growth occurs rapidly and thus seems to increase the need for specific nutrients to meet fetal needs, such as VA( Reference Ramalho and Dolinsky 14 ). Furthermore, it is in this trimester that the mother-to-child transfer of nutrients may occur more intensely, highlighting the necessity of achieving good fetal stores of VA in the liver in this period( Reference Ramalho and Dolinsky 14 , Reference Kæstel, Martinussen and Aaby 26 ). Thus, having a good nutritional status before the third trimester might prevent inadequate mother-to-child transfer of VA and/or insufficient storage of retinol in the fetal liver, which is essential to complement the VA supply provided by human milk in the first 6 months of life( Reference Ramalho and Dolinsky 14 ). Moreover, pre-pregnancy nutritional status has a crucial role in maintaining optimal concentrations of micronutrients throughout pregnancy, evidencing that optimal women’s nutritional status has to be reached before the establishment of pregnancy( Reference Ramakrishnan, Grant and Goldenberg 28 ).
In this study, average serum retinol at the second evaluation was higher than that at the first evaluation, with more than 75 % of our sample presenting retinol concentrations above 1·05 µmol/l at the second point measured in pregnancy, which is a cut-point to insufficiency in VA( 7 , Reference Ramalho and Dolinsky 14 ). It is important to note that plasma volume during pregnancy increases until the last trimester, consequently declining the concentration of nutrients, although it is less than the major changes in plasma volume( Reference King 29 ). Therefore, the total amount of micronutrients in circulation increases during pregnancy( Reference King 29 ).
In addition, the importance of VA liver stores to maintain the homoeostasis of serum retinol must be recognised. Under adequate VA nurture conditions, the liver is the main site of retinol storage in the body, and this regulates VA homoeostasis, keeping adequate concentrations of retinol in the blood stream. Furthermore, the VA status indirectly regulates bioconversion of carotenoids to retinol as well as the diet-responsive regulatory network( Reference King 29 ). Thus, even when the intake of VA does not match the recommendations, the serum retinol is influenced by the homoeostasis, bioconversion of carotenoids or even the inflammatory status( Reference Tanumihardjo, Russell and Stephensen 12 ). This fact may be one of the reasons our final multiple regression models have explained only 11·1 % of the variability in serum retinol. It is possible that majority of the women in our study had adequate pre-pregnancy nutritional status of VA, considering the mean values of retinol in both evaluations were higher than in other studies( Reference Kæstel, Martinussen and Aaby 26 , Reference Van Stuijvenberg, Schoeman and Nel 27 , Reference Hanson, Lyden and Abresch 30 ).
Previous surveys in other countries have shown different serum retinol concentrations before, during and after pregnancy. In the USA, the mean serum retinol in childbearing-age women was 1·79 (95 % CI 1·76, 1·81) μmol/l as reported by the National Health and Nutrition Examination Survey cycles of 2003–2004 and 2005–2006, and lower concentrations were associated with poverty and race (Hispanic and black)( Reference Hanson, Lyden and Abresch 30 ). In a large cross-sectional study of women within all gestational trimesters in Guinea-Bissau, the mean serum retinol was 1·03 (sd: 0·33) µmol/l, and the GA>20 weeks was consistently associated with approximately 0·1 µmol/l lower serum retinol (β=–0·09 to –0·11) compared with GA from 7 to 16 weeks( Reference Kæstel, Martinussen and Aaby 26 ). Another cross-sectional study of post-partum women in an impoverished area of South Africa, where pregnant women frequently consume liver in their diet, found a mean serum retinol of 1·03 (sd 0·4) µmol/l( Reference Van Stuijvenberg, Schoeman and Nel 27 ). These studies raise a few questions, such as the influence of pre-pregnancy nutritional status of VA, socio-economic level, food source availability and supplement use, among others. Also, our results reinforce the role of homoeostasis acting on serum retinol even in low-income areas, such as the Brazilian Amazon.
When considering the absorption rate and bioconversion factor to retinol, carotenoids are an important source of VA, especially for populations from low- and middle-income countries( Reference Penniston and Tanumihardjo 31 ). In Western Kenya, enhanced nutrition education and intake of orange-fleshed sweet potato by pregnant women through 9 months post-partum was positively associated with higher intakes of VA and reduced odds of low serum retinol binding protein, compared with participants who received standard-of-care approaches( Reference Girard, Grant and Watkinson 32 ). Amazonian fruits such as buriti and pupunha are extremely rich in carotenoids, either through the consumption of the fruit or their oil. Each 100 g of buriti has 4104 mg of carotenoids, while the same amount of pupunha has 1500 mg( 24 ). It is relevant to point out the availability of carotenoids and how these Amazonian fruits are usually consumed. Carotenoids from fruits are more available than vegetables owing to the weak layer in the fruit cells. In addition, heating up helps release carotenoids from their matrix( Reference Van Loo-Bouwman, Naber and Schaafsma 8 ). These two Amazonian fruits are normally cooked before consumption, which may foster improved nutrient availability. Experimental studies with rats have shown the improvement in serum and liver VA content with the consumption of buriti oil, which might be extended to humans( Reference Girard, Grant and Watkinson 32 , Reference Aquino, Vasconcelos and Pessoa 34 ).
The availability of foods and crops relates to intake and nutritional status of many nutrients, including VA. In our study, seasonality was one of the main factors positively associated with serum retinol. Seasonal patterns of vegetable and fruit production can potentially affect dietary VA intake( Reference Faber and Laubscher 35 ). This finding is in line with those of other studies, which showed that the time of year may affect the intake of nutrients, possibly due to the availability and affordability of food in some seasons( Reference Medeiros, Aquino and Soares 33 , Reference Aquino, Vasconcelos and Pessoa 34 ). In Manila, Philippines, a prospective study with young women showed that in specific periods, the consumption of fruits is higher than in others( Reference Beard, Murray-Kolb and Lawrence 36 ). Another prospective study in South Africa showed that the consumption of pumpkin, butternut squash and orange-fleshed sweet potato by pre-school-aged children was higher during rainy months( Reference Faber and Laubscher 35 ). The Amazon rainy season is the period of the year when Amazonian carotenoid-rich fruits are most available, enhancing the nutritional status of VA in our population( 24 ).
As expected, VA status at the first evaluation was a positive determinant of serum retinol concentrations in the following period assessed, evidencing that the retinol status in the first half of pregnancy influences serum retinol concentrations as pregnancy progresses (the half-life of serum retinol is nearly 10–36 h)( 37 ). A total of two major factors affect the regulation of retinol concentrations in blood: dietary VA and hepatic retinoids stored. Upon demand, these hepatic retinoids stores are mobilised by specific enzymes from hepatic retinoid stores, releasing retinol into circulation to ensure a constant supply to peripheral tissues, even under conditions where dietary retinoids are not available, either due to insufficient VA intake or due to pathological conditions( Reference Schreiber, Taschler and Preiss-Landl 38 , Reference Grumet, Taschler and Lass 39 ). Taking into account that most of our sample had serum retinol above 1·05 µmol/l, we considered the retinol stores were replete for majority of the participants. In addition, with the contribution of Amazonian fruits, the nutritional status of VA for most of our population was adequate, elucidating how important the nutritional status of VA is in the first half of pregnancy to foster good retinol levels in the final gestation period. This could offer a good supply of VA to the fetus before the last trimester, an important period of mother-to-child transfer of VA, as well as preclude clinical manifestation of VA deficiency (xerophthalmia) and its consequences, which are more often seen in the last trimester of pregnancy in low-income areas( 40 ).
Our results show that having a smoker in the house impacts negatively on serum retinol. Although our sample had a low prevalence of smoking during pregnancy within 16–20 weeks of pregnancy, passive smoking during pregnancy may affect the availability of antioxidants such as VA, which can affect fetal growth in either length or weight( Reference Titova, Ayvazova and Bichkaeva 41 ). Nicotine affects the role of retinoic acid in early embryo development through cellular stress( Reference Feltes, Poloni and Notari 42 ). In a national representative cross-sectional study in China, smoking habit during the second and third trimesters of gestation increased the odds of VA deficiency compared with those in the first trimester (second trimester: OR 2·4, 95 % CI 1·05, 5·46; third trimester: OR 2·92, 95 % CI 1·43, 5·93)( Reference Yang, Chen and Liu 43 ).
In this study, other covariates were not associated with the VA status in the final multiple model. In a cohort study in England, serum retinol in pregnancy was negatively associated with bone mineralisation in newborns( Reference Handel, Moon and Titcombe 5 ). Thus, considering the well-stablished role of vitamin D in the bone health( Reference Holick 44 ), we hypothesised that the VA and vitamin D might share metabolic pathways still unknown, which might influence the adequate nutritional status of both vitamins. However, we did not observe any association between these vitamins. Inadequate VA status in pregnancy is more likely to occur in less-advantaged populations, evidenced by studies in the USA( Reference Hanson, Lyden and Abresch 30 ) and Brazil( Reference Saunders, Leal and Neves 45 ). In addition, some episodes during the reproductive history of the pregnant women might affect their VA status, such as history of abortion, lowering the liver retinol stores for subsequent pregnancies( Reference Saunders, Leal and Neves 45 ). Despite it all, no association with VA status at mid pregnancy was verified for some covariates related to socioeconomic and reproductive aspects above mentioned. Furthermore, previous episodes of malaria can affect subject’s VA status, as during the reproductive cycle and metabolism of the Plamosdium malaria the host’s liver is affected. In this sense, frequent episodes of malaria through the life course may impair adequate retinol stores as well as raise concentrations of acute-phase proteins, such as CRP( Reference Barffour, Schulze and Coles 46 ). Nevertheless, neither the history of malaria nor the CRP concentration was associated with the VA status in pregnancy in this study.
Limitations of the study include the lack of data on other biochemical indicators of VA status, for example, retinol-binding protein; the FFQ did not include amounts of food intake, precluding estimates of nutrient intake by the participants and the fact that our study sample was not representative of all pregnant women living in both rural and urban areas, with losses to follow-up higher than expected, mainly because of difficulties in contacting participants to attend evaluations. As for strengths, the prospective study design allowed us to assess causality, the measurement of serum retinol used the HPLC assay recommended by WHO and all data and biomarker collection was done under very good quality conditions, ensured through periodic training. Thus, other populations with features similar to ours in the Brazilian Amazon can benefit from our results.
Conclusion
The results of this prospective cohort study revealed some predictors of VA status at commencement of the third trimester of pregnancy in the Western Brazilian Amazon. The positively associated factors were the consumption of Amazonian fruits, seasonality (Amazon winter, the rainy season) and serum retinol at the first evaluation; the negatively associated factor was having a smoker in the house, a proxy of passive smoking during pregnancy. Effective actions for improving the consumption of locally available pro-VA-rich fruits by pregnant women, as well as avoiding passive smoking during pregnancy, are potentially important interventions to improve VA status in Western Brazilian Amazonian pregnant women.
Acknowledgements
The authors are thankful to all participants and professional health workers involved in this study, as well as the Municipal Health Secretariat and all Basic Care Units of Cruzeiro do Sul. Members of MINA-Brazil Study Group: Alicia Matijasevich Manitto, Bárbara Hatzlhoffer Lourenço, Maíra Barreto Malta, Marly Augusto Cardoso, Paulo Augusto Ribeiro Neves (University of São Paulo); Suely Godoy Agostinho Gimeno (Federal University of São Paulo); Bruno Pereira da Silva, Rodrigo Medeiros de Souza (Federal University of Acre, Cruzeiro do Sul, Brazil); Marcia Caldas de Castro (Harvard T.H. Chan School of Public Health).
This work was funded by the National Counsel of Technological and Scientific Development – CNPq (grant no. 407255/2013-3), the Maria Cecília Souto Vidigal Foundation and the São Paulo Research Foundation – FAPESP (grant no. 2016/00270-6). P. A. R. N. received scholarships from the Brazilian Federal Agency for Support and Evaluation of Graduate Education – CAPES (grant no. PDSE – 88881.133704/2016-01). The funders had no role in the design, analysis or writing of this article.
P. A. R. N., B. H. L., M. C. C., M. A. C. conceived and designed the methods of the study. P. A. R. N., C. A. S. C. collected the data. P. A. R. N., M. B. M., M. A. C. analysed the data. P. A. R. N. wrote the first draft of the article, with critical revisions by M. A. C. All authors reviewed the manuscript content and have approved the final version submitted for publication.
The authors declare that there are no conflicts of interest.







