Population ageing is set to become one of the greatest transformations of the twenty-first century with far reaching implications for all sectors of society and it is estimated that by 2050 one in every five people will be aged 60 years or over(1). While most will be in good health, this population will be confronted with the potential impact of the ageing process which can result in a decreased quality of life, illness and disease(Reference Kirkwood2). Research over the past number of decades has shown that adequate nutritional status can play a key role in preventing or delaying the progression of age-related diseases such as CVD, reduced cognitive function and osteoporosis(Reference Pirlich and Lochs3–Reference Ruxton, Derbyshire and Toribio-Mateas7). However, physiological and social changes such as decreased food intake, impaired sensory perception, malabsorption, declining activity and increased disability increase the risk of inadequate nutritional status (particularly for micronutrients) in this population group(Reference Zhu, Devine and Suleska8, Reference Wysokiński, Sobów and Kłoszewska9). Further insights into the nutrient intake and diet quality of older adults may help to improve nutritional status and contribute to healthy ageing and overall quality of life for this population group. This review aims to investigate the nutrient intake and status of older adults in Europe and to explore the potential role of fortified foods and nutritional supplements in addressing some of the nutritional challenges in this age group.
Methods
Inclusion/exclusion criteria
This review includes nutrient intake and nutritional status data from either nationally representative food/nutrition surveys or studies of large cohorts of older adults (≥60 years but not <50 years) across Europe. For inclusion in this review, the studies were conducted post 2000 and dietary intake data were collected at an individual level via food records, 24 h recalls or diet history.
Comparison of studies
Data from nationally representative nutrition surveys in eighteen European countries and nine key studies in large cohorts of older adults across Europe are presented in this review. Across studies, there was a variation in methodologies used (e.g. methods used to collect food intake and biochemical data, reference cut-off points used to assess nutrient intakes and status and age-group cut-offs). Furthermore, for estimation of micronutrient intakes, some studies may underestimate intakes as they do not account for nutritional supplement use or collect detailed information on the consumption of fortified foods.
Estimation of nutritional quality of diets
Nutritional quality of diet in older adults across Europe is assessed in this review using the most recent dietary reference values (DRV) available. Mean intakes reported in studies are compared with recommended intakes (RI), population reference intakes (PRI) or adequate intakes (AI)(10). The RI is the range of intake for macronutrients (% of energy intake), that is adequate for maintaining health and associated with a low risk of selected chronic diseases while the PRI (or reference nutrient intake (RNI)) is the level of (nutrient) intake that is adequate for virtually all people in a population group assumed to meet the requirements of 97·5 % of the individuals in the population. The AI is the value estimated when a PRI cannot be established and is the average observed nutrient intake by a population group of apparently healthy people that is assumed to be adequate. At an individual level the estimated average requirement (EAR; the level of (nutrient) intake estimated to meet the requirements of 50 % of individuals) is used to assess the prevalence of inadequate intakes and this review will make reference to the %<EAR where mentioned within individual studies.
Macronutrients, dietary fibre and salt intakes
Total fat and individual fatty acids
Dietary fat is an important source of energy in the diet and fatty acids are essential components of cell membranes and pre-cursors for many signalling molecules. Research over the past number of decades has shown that imbalances in dietary fat intake are associated with the development of many chronic diseases(11). The European Food Safety Authority (EFSA) has recently set an RI for total fat of 20–35 % of energy (%E)(11), similar to the Nordic Nutrition Recommendations (NNR) RI of 25–40 %E(12). The UK Department of Health (UK DoH) has set a recommended maximum average population intake of 33 %E from total fat(13). Table 1 presents the mean intakes of macronutrients, including total fat, among older European adults (>50 years) reported in National Nutrition Surveys across Europe since 2000. Total fat provides on average 29 %E among older Portuguese adults in line with the UK average population intake recommendation of 33 %E; however, intakes in all other European countries exceed this recommendation with mean intakes ranging from 34 to 40 %E.
Table 1. Summary of mean intakes of macronutrients, sugars, dietary fibre and salt in older European Adults from National Nutrition Surveys

Elevated intakes of saturated fat are known to increase circulating LDL-cholesterol concentrations and the risk of CVD, while long-chain n-3 PUFA are known to confer benefits to metabolic health and low intakes of these essential fatty acids may contribute to age-related functional decline(Reference Sanders14–Reference Burlingame, Nishida and Uauy16). As SFA are synthesised by the body they are not required in the diet; hence, the EFSA has not set a DRV for saturated fat but recommends to keep intakes as low as possible(11). National guidelines from the UK and the NNR state that saturated fat intakes should not exceed 10 %E in the population(12, 13). Mean intakes of saturated fat among older adults across Europe exceed these recommendations with mean intakes ranging from 10 %E in Portugal to 17 %E in Belgium (Table 1). For MUFA and PUFA, the EFSA has not established a DRV; however, the NNR has set an RI of 10–20 %E for MUFA and 5–10 %E for PUFA(11, 12). The UK DoH has set a recommended minimum average population intake of 12 %E for MUFA and 6 %E for PUFA(13). Mean intakes of MUFA among older adults in Austria, Finland, Germany, Ireland, Norway, Portugal and Sweden are in line with the UK recommendation of 12 %E (Table 1). Intakes in other European countries are above this, ranging from 13 %E in Denmark to 17 %E in Italy and Spain. Mean intakes of PUFA among older European adults are generally in line with the UK recommendation of 6 %E (range 4–6 %E, Table 1) with the exception of Austria, Belgium and the Netherlands where PUFA provide 7 %E.
n-3 PUFA have been shown to reduce the risk of mortality from CHD and the EFSA has set an AI of 0·5 %E for α-linoleic acid and ≥250 mg/d for EPA and DHA combined(11). National nutrition surveys in Austria, Finland, the Netherlands and Ireland have reported mean intakes of α-linoleic acid and EPA + DHA in older adults above the AI from the EFSA (range 0·6–1·2 %E)(Reference Elmadfa, Hasenegger and Wagner17–Reference Li, McNulty and Tiernery20). However, a study among a convenience sample of community dwelling older adults in Southern France has reported that older adults have low median intakes of n-3 PUFA (α-linoleic acid: 0·4 %E, EPA + DHA: 195 mg/d)(Reference Carrière, Delcourt and Lacroux21).
Protein
The current recommendations for protein within Europe are based on nitrogen balance within the body and the EFSA has set a PRI of 61 and 55 g/d for men and women (≥60 years), respectively(22). The NNR has set an intake range for individuals of 15–20 %E from protein(12). National Nutrition Surveys across Europe have reported that protein provides 15–20 %E (67–89 g/d) in older adults (Table 1). A recent systematic review of macronutrient intakes of community dwelling older adults in Western populations reported that 10 % of adults aged over 60 years did not meet the EAR for protein set by the EFSA (0·66/kg bw/d) suggesting that a small proportion of older adults may be at risk of inadequate intakes of protein(Reference ter Borg, Verlaan and Mijnarends23). Studies in recent years have highlighted a possible need for increased protein requirements in older adults to prevent the loss of lean body mass, particularly muscle mass (sarcopenia)(Reference Cruz-Jentoft, Baeyens and Bauer24) in order to support optimal health and maintain functionality(Reference Bauer, Biolo and Cederholm25–Reference Granic, Mendonça and Sayer29). However, to date there has been insufficient evidence to update DRV for protein based on optimal health and muscle function in European countries. While a recent review in older adults ≥50 years globally has reported that the prevalence of sarcopenia is substantial in community dwelling older adults (1–29 %), the authors also concluded that protein supplements have not shown consistent benefits on muscle mass and function(Reference Cruz-Jentoft, Landi and Schneider30).
Carbohydrate
While the absolute dietary requirement for glycaemic carbohydrates is not known, energy balance is the ultimate goal. Hence, DRV for carbohydrate are set to meet energy needs in the context of acceptable intake levels of fat and protein(31). The EFSA and NNR currently recommend that carbohydrates provide 45–60 %E for individuals, and similarly the UK Scientific Committee on Nutrition (SACN) has set a recommended average population intake of at least 50 %E(32). National Nutrition Surveys across Europe have reported mean intakes of carbohydrate below the recommendation of 50 %E (Table 1) (range 39–48 %E).
Following recent reviews of evidence on carbohydrate intake and health, guidance on intakes of free sugars has been advised(32, 33). Free sugars include all mono- and di-saccharides added to foods by the manufacturer, cook or consumer, plus sugars naturally present in honey, syrups and unsweetened fruit juices. The WHO recommends that intake of free sugars should be <10 %E for individuals with a conditional recommendation of <5 %E (based on low to moderate evidence of a relationship between free sugar intake and body weight and dental caries)(33). The UK SACN recommends that the average population intake of free sugars should not exceed 5 %E (based on the effect of free sugar intake on the risk of dental caries and total energy intake)(32). Data on intakes of free sugars in older adults in Europe are limited; however, mean intakes of free sugars in older adults range from 5 %E in Spain to 11 %E in the UK and Netherlands (Table 1) with a moderate proportion of Spanish adults (75 % across all ages; exact proportion not provided for older adults alone) and just 31 % of Dutch adults adhering to the WHO guideline of <10 %E(Reference Ruiz, Rodriguez and Valero34, Reference Sluik, van Lee and Engelen35).
Inadequate intakes of dietary fibre can lead to impaired bowel function and constipation, which can adversely affect the quality of life and may contribute to increased risk of gastrointestinal disease. The EFSA has set an AI for all adults for dietary fibre of 25 g/d for normal bowel function(31) while the UK SACN recommends an average population intake of 30 g/d(32). The NNR recommend an intake of 30 g/d for men and 25 g/d for women(12). Mean intakes of dietary fibre among older adults in most European countries are below the current recommendations (Table 1) (range 15–23 g/d)(Reference Elmadfa, Hasenegger and Wagner17–Reference Ocké, Buurma-Rethans and de Boer19, 36–Reference Bates, Cox and Nicholson46). Intakes of dietary fibre are higher for older adults in Norway and Germany, 25 and 26 g/d, respectively, in line with the recommendation from the EFSA, but they are below the UK SACN and NNR recommended intakes.
Salt (sodium equivalents)
It is widely accepted that sodium intake is associated with blood pressure which can increase the risk of CVD. The WHO recommends that salt intake should be limited to 5 g/d, similar to country specific guidelines across Europe while the EFSA is currently reviewing evidence to set a DRV for sodium in European populations(12, 13, 47–Reference Strohm, Bechthold and Ellinger49). National Nutrition Surveys carried out across Europe have reported that intakes of salt from food sources (excluding discretionary salt) in older adults are lowest in Norway, the UK and Lithuania (3–6 g/d) while intakes in Germany, the Netherlands, Sweden, Andorra, Iceland, Austria, France and Denmark are as high as 7–9 g/d (Table 1). However, it is estimated that 25–30 % of salt intake is from discretionary sources(Reference Giltinan, Walton and Flynn50); hence, urinary sodium excretion is a more accurate reflection of true intake. Twenty-four-hour urinary sodium excretion values have been reported in adult populations in Finland (25–64 years), Italy (39–79 years), Portugal (65–79 years) and the UK (19–64 years) indicating a mean intake of 8–11 g salt daily in European adults(Reference Bates, Cox and Maplethorpe51–Reference Moreira, Sousa and Guerra54), well above the current recommendations.
Micronutrient intake and status
Adequate micronutrient status is important in preventing or delaying the progression of age-related diseases, such as CVD, reduced cognitive function and osteoporosis(Reference Pirlich and Lochs3–Reference Moore, Hughes and Ward6). While food intake and energy requirements decrease with increasing age, the requirements for micronutrients remain the same as for younger adults and this, in combination with a range of physiological and social changes, may place older adults at increased risk of micronutrient inadequacies.
Vitamin D
Vitamin D has long been recognised as a key nutrient for bone health. However, studies in recent years have suggested that vitamin D may be associated with other non-skeletal outcomes such as muscle strength and general health/lower disease risk(55–Reference Granic, Hill and Davies57). Older adults are at increased risk of vitamin D deficiency not only due to reduced dermal production but also because of age-related factors such as reduced food intake and possible decreased sun exposure(55). The EFSA has recently set an AI for vitamin D of 15 µg/d based on serum 25(OH)D status deemed to be adequate for bone health(58). Similar recommendations have been set for vitamin D across Europe (UK SACN: RNI 10 µg/d, NNR: recommended intake 10 µg/d) based on requirements for bone health(12, 56). Mean intakes of vitamin D among older European adults lie substantially below these recommendations (range 2–8 µg/d) with the exception of Iceland and Finland where mean intakes are approximately 11 µg/d (Table 2). For most European countries, it has been reported that over 90 % of older adults have intakes below 10 µg/d(Reference Elmadfa, Hasenegger and Wagner17, Reference Ocké, Buurma-Rethans and de Boer19, 36, Reference Lopes, Torres and Oliveira43, Reference Temme, Huybrechts and Vandevijvere59, Reference Olza, Aranceta-Bartrina and González-Gross60). The higher intakes observed in Finland and Iceland may be partly attributed to use of nutritional supplements but is more likely to be due to the routine fortification of fluid milks and fat spreads with vitamin D in these countries. Vitamin D status is measured by serum 25(OH)D concentrations with some variations in the thresholds used to define vitamin D deficiency e.g. UK SACN <25 nmol/l, US Institute of medicine <30 nmol/l(55, 56). Using the IOM threshold (<30 nmol/l), the prevalence of vitamin D deficiency in older adults has been reported in the Netherlands (14 %), Iceland (17 %), Germany (30 %) and Portugal (40 %) while the prevalence below the threshold of 25 nmol/l has been reported for older adults in Ireland (1 %), Sweden (1 %), the UK (13 %), Finland (15 %), Austria (20 %), Italy (22 %) and Spain (41 %)(Reference Elmadfa, Hasenegger and Wagner17, Reference Brouwer-Brolsma, Vaes and van der Zwaluw61–Reference Rodríguez Sangrador, Beltrán de Miguel and Quintanilla Murillas69). While a higher prevalence of vitamin D deficiency has been observed in Southern European countries this has previously been suggested to be attributable to more skin pigmentation and sun habits e.g. avoidance at peak times in these countries(Reference van Schoor and Lips70).
Table 2. Summary of mean intakes of micronutrients in older European Adults from National Nutrition Surveys
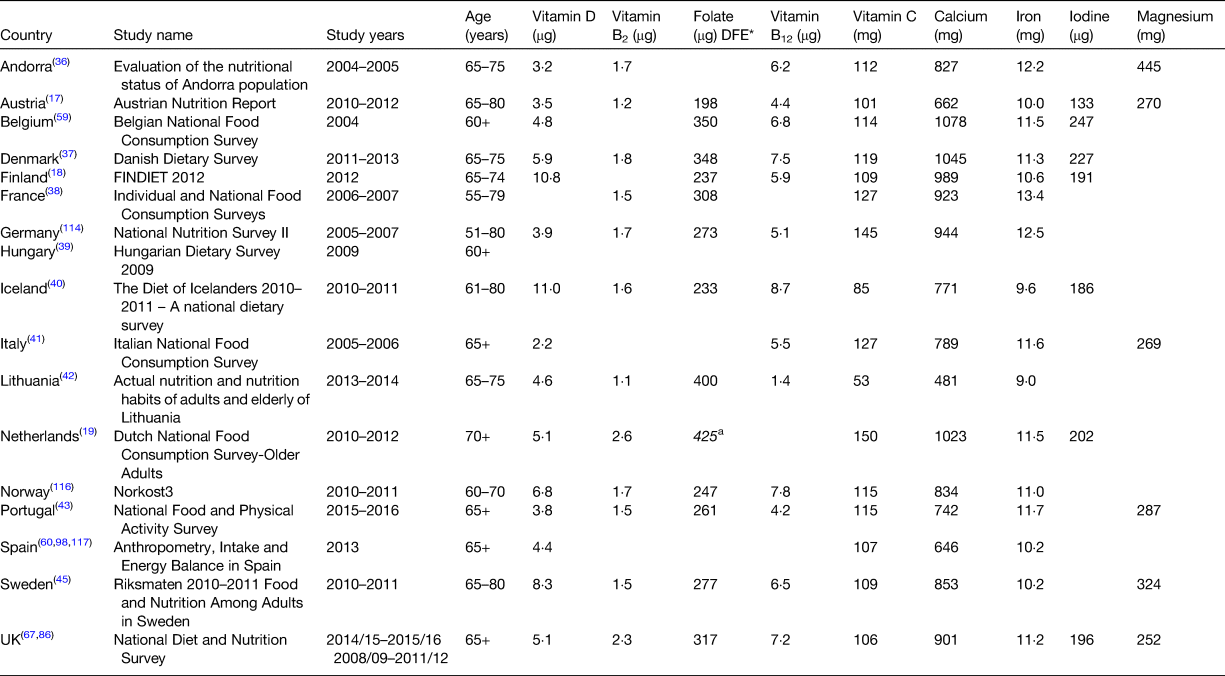
* Data reported as dietary folate equivalents (DFE).
Riboflavin
Riboflavin, in its coenzymatic forms, FMN and FAD, is required for numerous oxidation/reduction reactions and plays an integral role in the metabolism of other B-vitamins and the related metabolite homocysteine. Evidence has also shown that riboflavin plays a key role in the regulation of blood pressure in those with the 677TT MTHFR genotype and along with other B vitamins plays a role in slowing the progression of cognitive decline and possibly reducing the risk of depression in ageing(Reference Moore, Hughes and Ward6, Reference Wilcken, Bamforth and Li71). The EFSA has recently set a PRI for riboflavin of 1·6 mg/d (>18 years) based on body saturation measured through urinary riboflavin excretion(72). The mean intakes of riboflavin in older adults across Europe are typically close to or above this PRI (range 1·5–2·6 mg/d; with the exception of Lithuania and Austria: 1·1 and 1·2 mg/d) (Table 2). Approximately 1–25 % of older adults across Europe have been reported to have inadequate intakes of riboflavin when compared with the EAR(Reference Ocké, Buurma-Rethans and de Boer19, Reference Þorgeirsdóttir, Valgeirsdóttir and Gunnarsdóttir40, Reference Kehoe, Walton and Hopkins73, Reference Mensink, Fletcher and Gurinovic74) and although low intakes of riboflavin have been widely reported among older adults in Europe, there does not appear to be a large public health concern about this level of riboflavin intakes. Riboflavin status has not been widely reported among older European adults; however, the Irish National Adult Nutrition Survey and the UK National Diet and Nutrition Survey (UK NDNS) have recently reported that 52 and 29 % of older adults, respectively, have erythrocyte glutathione reductase activation coefficient (EGRac) values greater than 1·3 (which is indicative of low/deficient riboflavin status)(Reference Roberts, Steer and Maplethorpe67, Reference Kehoe, Walton and Hopkins75). The functional significance of these findings is unclear as use of EGRac as a biomarker has limitations(72).
Folate
Deficiencies in folate can result in impaired biosynthesis of DNA with clinical symptoms including the development of megaloblastic anaemia and neuropathy(76, Reference Koike, Takahashi and Ohyama77) and longer term low folate status has been associated with higher rates of cognitive impairment, CVD and cancer mortality(Reference Moore, Hughes and Ward6, Reference Peng, Dong and Wang78). Conversely, long-term exposure to high intakes of the synthetic form of folate (folic acid) is associated with the masking of vitamin B12 deficiency anaemia in older adults, allowing the associated irreversible neurological symptoms to progress(76, Reference Crider, Bailey and Berry79, 80). The UK DoH has set an RNI of 200 µg/d for total folate and the NNR has set a recommended intake of 300 µg/d for total folate(12, 13). The EFSA has recently set a PRI for dietary folate equivalents (DFE; which accounts for the higher bioavailability of folic acid: 1 µg DFE = 1 µg food folate + (1·7 × folic acid)) of 330 µg/d for all adults(76). While few European countries report mean intakes of DFE, the Dutch National Food Consumption Survey of Older Adults (DNFCS-older adults) has recently reported that older adults have mean DFE intakes of 425 µg/d which is higher than the PRI set by the EFSA(Reference Ocké, Buurma-Rethans and de Boer19) (Table 2). Mean intakes of total folate in most European countries meet the recommended intake from the NNR (200 µg/d) (Table 2), but were typically below the UK DoH RNI of 330 µg/d, with the exception of intakes in France, Denmark, Belgium and Lithuania (range 348–400 g/d). A recent review in older adults in the Western world reported that 29–35 % of older adults have inadequate intakes of folate when compared with the EAR from the NNR of 200 µg/d(Reference ter Borg, Verlaan and Hemsworth81). Serum and erythrocyte folate concentrations are sensitive biomarkers of folate intake and status and the EFSA recommends that measurements below both 340 nmol/l for erythrocyte folate and <10 nmol/l for serum folate are required to indicate folate deficiency(76). Mean status measurements of erythrocyte folate have been reported in the UK NDNS (585 nmol/l)(Reference Roberts, Steer and Maplethorpe67) and in other key studies of older adults in Europe including the Newcastle 85+ study in the UK, the Irish Longitudinal Study on Ageing (TILDA) in Ireland and the ZENITH study across three European countries with measures ranging from 856 to 1087 nmol/l(Reference Mendonça, Mathers and Adamson82–Reference Polito, Intorre and Andriollo-Sanchez84). Mean serum folate has been reported to range from 18·1 nmol/l in the UK NDNS to 24·5 nmol/l in the KORA-Age Study in Germany(Reference Roberts, Steer and Maplethorpe67, Reference Conzade, Koenig and Heier85). The mean values reported for erythrocyte folate and serum folate in older adults across Europe are above the cut-offs proposed by the EFSA and studies in older adults across the UK and Europe have found no evidence of folate deficiency(Reference Mendonça, Mathers and Adamson82, Reference Polito, Intorre and Andriollo-Sanchez84, Reference Bates, Lennox and Prentice86). While a recent study from the TILDA in Ireland has indicated that a high proportion of older adults (15 %) have low or deficient folate status using plasma folate measurements; a single measurement of serum/plasma folate is not thought to be as informative for the assessment of folate status and body stores as this is a sensitive marker of recent dietary intake and is subjected to prandial variation(Reference Green87).
Vitamin B12
Low/deficient vitamin B12 status has been widely reported in older adults despite adequate dietary intakes; however, the maintenance of an optimal status of vitamin B12 is not only dependent on adequate dietary intake but more critically on effective absorption which diminishes with age(Reference Allen88, Reference Hughes, Ward and Hoey89). Apart from the clinical features of vitamin B12 deficiency (megaloblastic anaemia and irreversible neuropathy), emerging evidence indicates that subclinical deficiency (low biomarker status) may be implicated in the development of several chronic age-related diseases with some evidence to suggest that suboptimal levels of B12 or folate can raise homocysteine which is significantly associated with risk of bone fractures(Reference van Wijngaarden, Doets and Szczecińska90) and risk of CVD(Reference Jacobsen91). The EFSA has recently set an AI of 4 µg/d for adults based on biochemical markers of adequate cobalamin (vitamin B12) status(92) while the UK DoH has set an RNI of 1·5 µg/d and the NNR has set a recommended intake of 2 µg/d(12, 13). Intakes of vitamin B12 in older adults across Europe are lowest in Lithuania (1·4 µg/d) but typically range from 4·2 to 8·7 µg/d in other European countries which is above the AI proposed by the EFSA (Table 2). Serum vitamin B12 has been the most widely reported measurement of vitamin B12 status both in nationally representative surveys and other cohorts across Europe with variations in the cut-offs used to assess deficiency (<148/150 and <185 pmol/l). Mean serum vitamin B12 values have been reported to range from 232 to 324 pmol/l in studies of older adults in the UK, Germany and the Netherlands(Reference Roberts, Steer and Maplethorpe67, Reference Mendonça, Mathers and Adamson82, Reference Conzade, Koenig and Heier85, Reference Brouwer-Brolsma, Dhonukshe-Rutten and van Wijngaarden93). Small proportions of older adults (<7 %) in the UK NDNS and the TILDA study in Ireland have been reported to have deficient vitamin B12 status (<148/150 pmol/l)(Reference Roberts, Steer and Maplethorpe67, Reference Laird, O'Halloran and Carey83) with up to 12 % of Irish adults reported to have low/deficient status (<185 pmol/l)(Reference Laird, O'Halloran and Carey83). However, a higher proportion (17 %) of those aged 85+ in the Newcastle 85+ study in the UK have been reported to have deficient vitamin B12 status (<148 pmol/l) which may be due to methodological differences between the studies but may also highlight the potential impact of age on vitamin B12 status(Reference Mendonça, Mathers and Adamson82). As vitamin B12 absorption becomes less efficient with ageing it is important to ensure AI in older adults to prevent increased risk of deficiencies.
Vitamin C
Vitamin C acts as an antioxidant within the body through its role as an enzyme cofactor for biochemical reactions catalysed by oxygenases and plays an important role in the biosynthesis of collagen(94). Some studies have reported a link between low vitamin C intake and an increased risk of CVD in adults of all ages and increased risk of frailty in older adults(Reference Knekt, Ritz and Pereira95–Reference Balboa-Castillo, Struijk and Lopez-Garcia97). Age-related decreases in antioxidant enzyme activity are reported to contribute to increased oxidative damage and age-related degeneration highlighting the need for continuous supply of key antioxidant nutrients. The EFSA has set a PRI for vitamin C of 110 mg/d for men and 95 mg/d for women while the recommended intake from the NNR is 75 mg/d(12, 94). Mean intakes of vitamin C among older adults in Europe are lowest in Lithuania (53 mg/d) and Iceland (85 mg/d) and typically range from 101 to 150 mg/d in other European countries which is above the recommended intake from the NNR and PRI from the EFSA (Table 2). However, it has been reported that 10–35 % of older adults have intakes below the respective EAR in the Netherlands, Portugal, Spain and Iceland(Reference Ocké, Buurma-Rethans and de Boer19, Reference Þorgeirsdóttir, Valgeirsdóttir and Gunnarsdóttir40, Reference Lopes, Torres and Oliveira43, Reference Olza, Aranceta-Bartrina and González-Gross98).
Calcium
Calcium is well recognised for its vital structural role in the skeleton and the key role within nerve transmission and intracellular messaging in the body. The EFSA has recently set a PRI of 950 mg/d for adults based on calcium balance data (intake–excretion, excluding dermal losses)(99). While the functional outcome of inadequate calcium intake is often reduced bone density, the EFSA has recommended that more research is needed on the effects of very old age (>80 years) on calcium requirements (including measurements of efficiency of absorption, obligatory losses and changes in bone calcium content)(99). The UK DoH and NNR have set a recommended intake of 700 and 800 mg/d, respectively for calcium(12, 13). Mean intakes of calcium in older adults in Lithuania, Austria, Iceland, Italy, Andorra, Germany, Norway, Portugal and Sweden are low when compared with the PRI proposed by the EFSA (range 481–853 mg/d) (Table 2). While intakes in France, the UK, Finland, Belgium, the Netherlands and Denmark are more in line with the recommendation (901–1078 mg/d). Despite these intakes, up to 50 % of older adults across Europe have been reported to have inadequate intakes of calcium when compared with an EAR of 525 or 750 mg/d (ranging from <5 % in Spain and the Netherlands to 50–56 % in Portugal and Poland)(Reference Lopes, Torres and Oliveira43, Reference Mensink, Fletcher and Gurinovic74).
Iron
Iron is required within the body for transport of oxygen and is an essential component of many enzymes playing a role in electron transport, respiration and hormone synthesis. Iron deficiency anaemia is the clinical manifestation of low iron intake and status and has been associated with numerous health implications in older adults including a decline in physical performance, cognitive impairment, increased susceptibility to falling and frailty and mortality(Reference Price, Mehra and Holmes100). The EFSA has recently set a PRI for iron of 11 mg/d for men ≥18 years and postmenopausal women based on requirements to replace whole-body losses(101). The UK DoH has set an RNI of 8·7 mg/d for iron and the NNR has set a recommended intake of 9 mg/d(12, 13). Mean intakes of iron among older adults in Europe range from 9·0 to 12·2 mg/d (Table 2), above the recommendations from the UK and NNR and are approaching or above the PRI from the EFSA. While 10–50 % of older adults have been reported to have inadequate intakes of iron in individual studies (highest among females), the prevalence of iron deficiency anaemia is very low in this age group across Europe (<5 %)(Reference Elmadfa, Hasenegger and Wagner17, Reference Roberts, Steer and Maplethorpe67).
Iodine
Iodine is an important mineral for health, required for the production of key thyroid hormones thyroxine and triiodothyronine, which are essential for cellular metabolism, growth and physical development. While the crucial role of iodine at important developmental stages is widely known much less is known regarding its impact throughout the lifespan and in particular for the older population. A recent follow-up to a birth-cohort in a Scottish population exploring iodine exposure on brain structures and cognition in older people found an association between low iodine intake and inner brain atrophy, raising an important hypothesis which warrants further studies(Reference del, Valdés Hernández and Kyle102). The EFSA has recently set an AI for iodine of 150 µg/d for adults based on the urinary iodine excretion concentration accepted as indicating sufficient iodine intake (associated with the lowest prevalence of goitre in children, in the absence of data for adults)(103). Mean intakes of iodine in older adults across Europe (Table 2) are lowest in Austria (133 µg/d) but typically range from 186 to 247 µg/d which is above the AI proposed by the EFSA. The WHO have defined a median urinary iodine of 100–199 µg/l as optimal for iodine nutrition and urinary iodine values have been reported for older adults in Ireland (median: 101 µg/l) and Austria (mean: 110 µg/l)(Reference Elmadfa, Hasenegger and Wagner17, Reference McNulty, Nugent and Walton104, Reference de Benoist, Andersson, Elgli, Takkouche and Allen105). It has previously been reported that iodine intakes can be directly influenced by seasonal changes in agricultural practices and so it is important to continue to monitor intakes and status in older adults to ensure sufficiency.
Magnesium
Magnesium plays a key role in skeletal development and in the maintenance of electrical potential in nerve and muscle membranes(13) and recent studies have suggested that magnesium could also be associated with muscle function in older adults and with risk of CVD in the general population(Reference Dominguez, Barbagallo and Lauretani106, Reference DiNicolantonio, O'Keefe and Wilson107). The EFSA has recently set an AI for magnesium of 350 mg/d for men and 300 mg/d for women based on observed intakes in healthy populations of the European Union(108). Mean intakes of magnesium in older adults from the UK, Italy, Austria and Portugal range from 252 to 287 mg/d which is below the AI and so the adequacy of intakes cannot be determined, while intakes in Sweden and Andorra are above the AI (324 and 445 mg/d, respectively) indicating there may be a low prevalence of inadequate intakes in these countries (Table 2). However, a recent review of micronutrient intakes in community dwelling older adults in the Western world reported that 73 % of men and 41 % of women had inadequate magnesium intakes when compared with the EAR from the NNR (men: 350 mg/d, women: 265 mg/d)(Reference ter Borg, Verlaan and Hemsworth81).
The role of fortified foods and nutritional supplements
Further research is necessary to identify the dietary patterns that are contributing to the micronutrient imbalances identified in older adults and to evaluate the best dietary strategies to ensure nutrient adequacy. Dietary strategies to improve micronutrient intakes and status may include food fortification (mandatory or voluntary) and/or the use of nutritional supplements. Mandatory fortification is that which is required by statute or officially encouraged (e.g. vitamin D fortification of fluid milks or folic acid flour fortification) while voluntarily fortification is at the discretion of the manufacturer to improve the nutrient profile of a food (e.g. the addition of vitamins and minerals to ready-to-eat breakfast cereals). Many countries in Europe recommend vitamin D supplements for older adults(12, 48, 56, 58, 109). Where data are available (UK, Ireland, the Netherlands, Germany, Austria and Finland), the consumption of fortified foods and nutritional supplements has been shown to be associated with higher dietary intakes and nutritional status in older adults, particularly for vitamin D and B-vitamins (with smaller contributions to vitamins C, E, calcium and iron)(Reference Ocké, Buurma-Rethans and de Boer19, Reference Roberts, Steer and Maplethorpe67, Reference Laird, O'Halloran and Carey83, Reference Conzade, Koenig and Heier85, Reference Bates, Lennox and Prentice86, Reference Moore, Hughes and Hoey110–Reference Jääskeläinen, Itkonen and Lundqvist113).
The contribution of nutritional supplements to the diets of older adults
Nationally representative surveys in the UK and the Netherlands have reported mean intakes of micronutrients in older adults from food sources only (excluding nutritional supplements) and from all sources (including nutritional supplements) allowing for calculation of the contribution of nutritional supplements to nutrient intake(Reference Ocké, Buurma-Rethans and de Boer19, Reference Bates, Lennox and Prentice86). The UK NDNS has reported that 41 % of older adults took a nutritional supplement with nutritional supplements making significant contributions to intakes of vitamins D (41 %), C (21 %) and B-vitamins (11–31 %) and smaller contributions to intakes of iron (9 %) and calcium (5 %)(Reference Roberts, Steer and Maplethorpe67, Reference Bates, Lennox and Prentice86). Similarly, the DNFCS-older adults survey has reported that 45 % of older Dutch adults take nutritional supplements making significant contributions to intakes of vitamins D (21 %), C (30 %) and B-vitamins (35–47 %) with smaller contributions to iron (11 %) and calcium (6 %)(Reference Ocké, Buurma-Rethans and de Boer19). At the time of the DNFCS-older adults survey there was a vitamin D supplementation policy for older adults; however, just 26 % of older women and 18 % of older men reported taking a vitamin D supplement(Reference Ocké, Buurma-Rethans and de Boer19). Data from Dutch participants in the NU-AGE study (65–80 years) reported significant contributions of nutritional supplements to intake of vitamins D (41 %), B6 (43 %), B12 (48 %) and total folate (19 %) with small contributions to intake of iron (12 %) and calcium (6 %)(Reference Berendsen, van Lieshout and van den Heuvel111). The TILDA study in Ireland investigated the determinants of vitamin B12 and folate status in older Irish adults (≥50 years) and found that nutritional supplement users had a significantly lower prevalence of low/deficient B12 or folate status than non-supplement users with nutritional supplement use being the largest predictor of status in this population(Reference Laird, O'Halloran and Carey83). The Ageing Cohort Study in Ireland also showed that biomarker status of vitamins B2, B6, B12 and folate were significantly higher in supplement users to non-users (>60 years)(Reference Moore, Hughes and Hoey110). Similar findings have been reported in the KORA-Age study in Germany (65–90 years) where no/irregular supplement use was a strong predictor of deficient vitamin D, B12 and folate status and in a convenience sample of older adults in Austria (70–90 years) where regular use of supplements significantly improved biochemical status for vitamins D, E, C and B-vitamins(Reference Conzade, Koenig and Heier85, Reference Fabian, Bogner and Kickinger112).
The contribution of fortified foods to the diets of older adults
To date the DNFCS-older adults survey is the only nationally representative survey which provides information on nutrient intakes from fortified foods(Reference Ocké, Buurma-Rethans and de Boer19). This study reported that 70 % of older adults consumed fortified foods and fortified foods made significant contributions to intakes of vitamins D (18 %), folate (18 %), B6 (17 %) and E (15 %) with smaller contributions to intakes of vitamins C, B1, B2, calcium and iron (4–6 %)(Reference Ocké, Buurma-Rethans and de Boer19). Data from Dutch participants in the NU-AGE study (65–80 years) has shown that fortified foods make important contributions to intakes of vitamin D (18 %) and B6 (10 %)(Reference Berendsen, van Lieshout and van den Heuvel111). Furthermore, data from the Ageing Cohort Study in Ireland showed that biomarker status of vitamins B2, B6, B12 and folate significantly increased with increasing intake of fortified foods(Reference Moore, Hughes and Hoey110).
A recent study in Finnish adults (including older adults) has demonstrated the benefits of a food fortification policy to improve nutritional status at a population level(Reference Jääskeläinen, Itkonen and Lundqvist113). The authors examined vitamin D status in older adults both pre- and post-national fortification policy and found that mean serum 25(OH)D concentration significantly increased (46 nmol/l v. 66 nmol/l) and the prevalence of vitamin D insufficiency significantly decreased (57 % v. 7 %) over the 11 year period which can be explained mainly by food fortification. However, the authors did note that there was also an increase in vitamin D supplement users (11 % v. 41 % in the total population >30 years old) over the time period which may also contribute to the increased status(Reference Jääskeläinen, Itkonen and Lundqvist113).
Conclusions
Adequate nutritional status plays an important role in preserving health status and preventing or delaying the progression of age-related diseases in older adults. Data from studies of older adults in Europe have highlighted unfavourable intakes of total and saturated fat, sugar, salt and dietary fibre with low intakes and suboptimal status of key micronutrients such as vitamins D, B2, B12, folate and calcium. Fortified foods and nutritional supplements have been shown to make significant contributions to intakes and status of these micronutrients in older adults; however, it is important to continue monitoring nutrient intakes and status in light of changing fortification practices and food consumption patterns. Future strategies to address the nutritional issues identified in older adults could include the promotion of healthy food choices (in line with dietary guidelines) and improvements of the food supply including reformulation (fat, sugar and salt), food fortification or supplementation with specific nutrients to promote successful ageing of our populations.
Acknowledgements
The authors would like to thank the Irish section of the Nutrition Society for inviting the present review paper as part of the postgraduate review competition.
Financial Support
This work was supported by funding from the Irish Department of Agriculture Food and the Marine.
Conflict of Interest
None.
Authorship
L. K. contributed to the design of the study, data analyses and wrote the first draft. All authors contributed to the writing of the final manuscript. All authors critically reviewed the manuscript and approved the final version submitted for publication.





