Introduction
The East Pacific green turtle Chelonia mydas occurs along the west coast of the Americas from central Baja California and the Gulf of California to northern Chile and also on island groups such as the Revillagigedos and Galapagos Archipelagos (Marquez, Reference Marquez1990). During El Niño years green turtles may stray as far north as British Columbia and south to Chile (Marquez, Reference Marquez1990; Chandler, Reference Chandler1991; Quiñones et al., Reference Quiñones, Gonzalez Carmen, Zeballos, Purca and Manzan2010). As no regional assessment exists for this stock East Pacific green turtles are included in global green turtle assessments, categorized as Endangered on the IUCN Red List (IUCN, 2010) and included in Appendix I of CITES (CITES, 2010).
The East Pacific green turtles found along the coasts of the Baja California peninsula come mainly from nesting beaches in Michoacán, the Revillagigedos Archipelago and Tres Marias Islands (Zweifel, Reference Zweifel1960; Brattstrom, Reference Brattstrom1982; Awbrey et al., Reference Awbrey, Leatherwood, Mitchell and Rogers1984; Alvarado-Diaz & Figueroa, Reference Alvarado-Diaz and Figueroa1990; Marquez, Reference Marquez1990; NMFS–USFWS, 2007). The coastal waters of Baja California Sur include some of the most important foraging grounds for this species and our study site, San Ignacio lagoon, is among the most important (Seminoff, Reference Seminoff2000; Gardner & Nichols, Reference Gardner and Nichols2001; Nichols, Reference Nichols2003; Seminoff et al., Reference Seminoff, Resendiz and Nichols2002, Reference Seminoff, Jones, Resendiz, Nichols and Chaloupka2003; Koch et al., Reference Koch, Brook and Nichols2007; NMFS–USFWS, 2007). Because of their high primary and secondary productivity these areas are also important fishing grounds for many small-scale fisheries targeting a variety of fin- and shellfish (SAGARPA, 2007).
Bycatch, the unintentional capture of non-target species whilst fishing (Soykan et al., Reference Soykan, Moore, Zydelis, Crowder, Safina and Lewison2008), has become a major issue in industrial (FAO, 2004; Gilman et al., Reference Gilman, Zollett, Beverly, Nakano, Davis and Shiode2006; Lewison & Crowder, Reference Lewison and Crowder2007; Casale, Reference Casale2008; Soykan et al., Reference Soykan, Moore, Zydelis, Crowder, Safina and Lewison2008) and artisanal fisheries (Godley et al., Reference Godley, Gücü and Broderick1998; Lagueux, Reference Lagueux1998; Koch et al., Reference Koch, Nichols, Peckham and de la Toba2006; Peckham et al., Reference Peckham, Diaz, Walli, Ruiz, Crowder and Nichols2007, Reference Peckham, Maldonado-Diaz, Koch, Mancini, Gaos, Tinker and Nichols2008), especially because it affects long-lived, slow-growing species such as marine mammals, sea birds and turtles (Lewison et al., Reference Lewison, Freeman and Crowder2004a; Soykan et al., Reference Soykan, Moore, Zydelis, Crowder, Safina and Lewison2008) and has negative effects on population viability (Heppell et al., Reference Heppell, Caswell and Crowder2000). Many bycatch studies are limited by the scarcity of direct observations (Soykan et al., Reference Soykan, Moore, Zydelis, Crowder, Safina and Lewison2008; Tomás et al., Reference Tomás, Gozalbes, Raga and Godley2008) and small spatial coverage (Lewison et al., 2004b; Soykan et al., Reference Soykan, Moore, Zydelis, Crowder, Safina and Lewison2008), which can only partially be solved using interviews with fishers (Carreras et al., Reference Carreras, Cardona and Aguilar2004; Carranza et al., Reference Carranza, Domingo and Estrades2006; Mejuto et al. Reference Mejuto, De la Serna, Valeiras and Camiñas2006).
Stranding data has been used in many studies as an index for turtle bycatch (Caillouet et al., Reference Caillouet, Shaver, Teas, Nance, Revera and Cannon1996; Epperly et al., Reference Epperly, Braun, Chester, Cross, Merriner, Tester and Churchill1996; Bugoni et al., Reference Bugoni, Krause and Petry2001; Lewison et al., Reference Lewison, Crowder and Shaver2003; Peckham et al., Reference Peckham, Maldonado-Diaz, Koch, Mancini, Gaos, Tinker and Nichols2008; Tomás et al., Reference Tomás, Gozalbes, Raga and Godley2008; Casale et al., Reference Casale, Affronte, Isacco, Freggi, Vallini and d’Astore2010), or other mortality factors (Chaloupka et al., Reference Chaloupka, Work, Balazs, Murakawa and Morris2008). However, bycatch is usually underestimated because fishing gear often does not leave marks on turtles. Furthermore, strandings only represent a small percentage of real mortality and quantifying total mortality can be difficult because stranding probability depends on currents, winds and distance from shore, all of which can substantially affect the number of carcasses on a specific beach (Epperly et al., Reference Epperly, Braun, Chester, Cross, Merriner, Tester and Churchill1996; Hart et al., Reference Hart, Mooreside and Crowder2006).
Here we combined a mortality census and interviews with local fishers to estimate the impact of the artisanal guitar-fish (Rhinobatus sp.) fishery in San Ignacio, Mexico, on marine turtles. We also attempted to evaluate total at-sea mortality, using drifters to quantify stranding probabilities of dead turtles in the lagoon.
Study area
The c. 110,000 ha San Ignacio lagoon is located in the Vizcaino Biosphere Reserve on the Pacific coast of the state of Baja California Sur, Mexico. A main channel connects the lagoon to the Pacific Ocean. Tides are mixed, with an average range of 1.6 m (CIBNOR, 1994). The lagoon is shallow with an average depth of < 3 m and hosts at least 85 species of algae and sea grasses (Delgadillo et al., Reference Delgadillo, Peinado, De la Cruz, Martinez-Parras, Alcaraz and De la Torre1992). For the purposes of this study the area was divided into three regions according to the shape and depth of the lagoon (upper, middle, lower; Fig. 1).
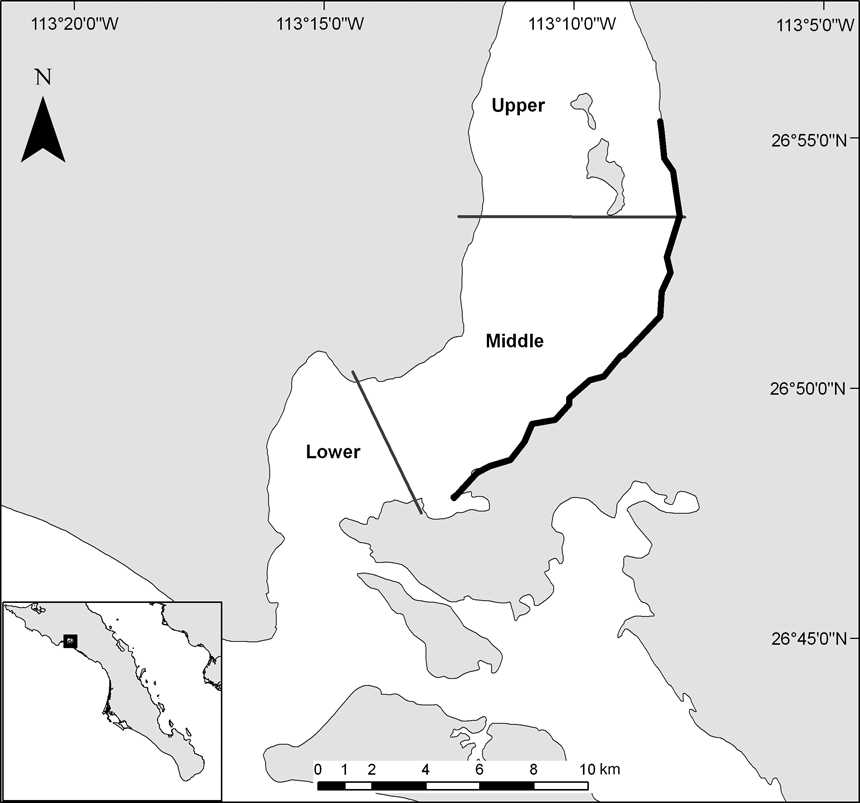
Fig. 1 The study area in the San Ignacio Lagoon in the Vizcaino Biosphere Reserve, indicating the 14 km of beach that was monitored during 2006–2008 (thick black line) and the division of the lagoon into upper, middle and lower regions. The inset indicates the location of the main map on the Pacific coast of the state of Baja California Sur, Mexico.
Methods
Fisheries sector
A total of nine cooperatives including 171 fishers (SAGARPA, 2007) and five independent fishers were officially registered in 2006 and 238 permits were expedited, of which 175 (75%) were for fisheries outside the lagoon (SAGARPA, 2007). There are three main seasons for fishermen in the lagoon: (1) whale-watching (January–April), when no nets are allowed because grey whales Eschrichtius robustus enter the lagoon; (2) the California halibut Paralichthys californicus/guitar-fish season (May–August), when 8-inch mesh monofilament bottom-set mesh-nets are used and checked every 24 hours; they are usually set outside the lagoon (because of permit regulations) but some illegal fishing occurs in the lagoon that has been suspected to cause high bycatch mortality of turtles; (3) lobster (Palinurus sp.) season (September–December) when lobsters are caught with box traps inside the lagoon. During the lobster season, fishers without a lobster permit usually fish outside the lagoon with nets and/or longlines or dive for pen-shells (Pinna rugosa and Atrina maura) in the lagoon.
Mortality data
From March 2006 to August 2009 mortality censuses were conducted first bimonthly (2006–2007) and then monthly (2008–2009) along a 10-km beach (13.5 km during the fishing season in June 2008 and 2009) on the eastern side of the lagoon (Fig. 1). During the main fishing season we conducted daily censuses of the beach for a total of 5, 6, 42 and 38 days in 2006, 2007, 2008 and 2009, respectively. One survey was conducted on each sampling occasion; walking the beach took 2.5–4 hours. The beach is only 5–20 m wide and dead turtles are easy to detect. Detection rate was thus close to 100% during daily censuses in June 2008–2009 but may have been less during the monthly or bimonthly census as scavengers could drag small carcasses away from the beach. We could not quantify their impact but from our observations scavengers such as coyotes Canis latrans and vultures would usually feed on the beach. We identified every carcass encountered, measured curved carapace length (CCL), recorded the location with a global positioning system and, when possible, established the cause of mortality based on examination of the carcass (Koch et al., Reference Koch, Nichols, Peckham and de la Toba2006). Every dead turtle was marked with spray paint and removed from the beach to avoid double counting.
During the guitar-fish fishery in June 2008 and 2009 we conducted daily at-sea monitoring (for a total of 30 and 28 days in June 2008 and June 2009 respectively, see below). Floating turtle carcasses were hauled aboard for species identification, measuring, and to determine cause of death. When possible, carcasses were marked with a plastic tag, screwed to the shell to avoid loss, and put back in the water. Locations of release sites were recorded to see when, where, and if a carcass would strand along the monitored beach. Annual stranding rate was calculated as the number of dead turtles per kilometre of monitored beach per year assuming a detection probability of 99% of carcasses on the beach for the entire study period.
Fishing effort
During the at-sea monitoring in June 2008 and June 2009 (see above) we monitored daily fishing effort in the lagoon from a small skiff with outboard motor. The survey was from the top to the mouth of the lagoon, usually passing along the central channel. When a halibut net was spotted (illegal inside lagoons in Mexico), we recorded its position, measured the total length and checked the number of entangled turtles. Fishing effort was calculated as the total length of nets per day. When the total length of a net was not available, as fishermen sometimes only have a buoy on one end of the net, we used mean length of the observed nets. Density of nets was calculated using a Kernel density estimator to highlight high-use areas within the lagoon (Worton, Reference Worton1989). The bottom-set nets are checked only every 24 hours and therefore the mortality rate for captured turtles is close to 100% because turtles cannot survive being entangled for longer than c. 60 minutes. As a result all turtles we found in the nets during these surveys were dead.
Interviews
From March 2006 to July 2008, 22 semi-structured interviews were carried out at five fishing camps (13% of regular fishers operating in the area; SAGARPA, 2007) along the east side of the lagoon. The interviews were to gather quantitative and qualitative data on fishing practices inside the lagoon and interactions between fisheries and turtles. Participants were chosen opportunistically and interviewed separately to avoid collecting group opinions (Janis, Reference Janis1972). Before each interview the purpose of the study was explained to each participant and anonymity was granted. Control questions were used to verify the consistency of the information obtained (for further details see Mancini & Koch, Reference Mancini and Koch2009). If not otherwise specified, turtles caught in nets were all dead; no fisher mentioned releasing live turtles.
Exploratory analysis: stranding probabilities and rates
In an attempt to estimate total at-sea mortality of green turtles we conducted a pilot study using marked drifters (oranges) during June 2008 and June 2009. Oranges float in the water column similarly to turtles, are not affected much by the wind and follow surface currents (Muhlin et al., Reference Muhlin, Engel, Stessel, Weatherbee and Brawley2008). Marked oranges were released from the top to the mouth of the lagoon along a transect in the central channel where most of the fishing occurred. We monitored the beach to detect stranded drifters from day 1 after deployment until we didn’t find stranded drifters for two consecutive days (day 7). As stranding rates can vary depending on the currents in the lagoon we deployed drifters during tidal conditions similar to those when the fishing occurred, usually 2–3 days around quarter moon tides. Drifter stranding rate was calculated as the total number of recovered drifters/total number of released drifters. We then estimated the stranding probability of carcasses that were found floating in the lagoon as a linear function of drifter stranding probability, including the following assumptions: (1) despite their different size and shape, turtle carcasses and drifters follow surface currents in a similar way; (2) oranges are likely to strand faster as they do not sink for a certain time as turtle carcasses do; (3) we considered a detection probability of 95% for oranges (high visibility because of their bright colour but a small probability that they become buried under sea-grass) and 99% for turtles (because of their larger size).
Assuming that the stranding probability of dead turtles was normally distributed, the overall regression model was:
where ![]() and
and ![]() are 12×1 vectors of stranding probabilities of dead turtles and drifters, respectively, β the regression coefficient associated with the probability of stranding of drifters and ε an independent random variable (Appendix 1). A confidence interval for the stranding probability of dead turtles was computed via parametric bootstrapping: the original vector of stranding probabilities of drifters was re-sampled 1,000 times by randomly sampling from the original dataset and the mean was calculated for each new vector obtained. The 25th and 975th largest values were used to obtain the bounds of a 95% bootstrapped confidence interval (Davis & Hinkley, Reference Davis and Hinkley1997).
are 12×1 vectors of stranding probabilities of dead turtles and drifters, respectively, β the regression coefficient associated with the probability of stranding of drifters and ε an independent random variable (Appendix 1). A confidence interval for the stranding probability of dead turtles was computed via parametric bootstrapping: the original vector of stranding probabilities of drifters was re-sampled 1,000 times by randomly sampling from the original dataset and the mean was calculated for each new vector obtained. The 25th and 975th largest values were used to obtain the bounds of a 95% bootstrapped confidence interval (Davis & Hinkley, Reference Davis and Hinkley1997).
Total mortality estimates
Total bycatch mortality was estimated from data on strandings and information collected through interviews. The actual number of dead turtles from stranding observations was estimated using:
where n was the number of dead turtles found on the beach during the study period. A confidence interval for the number of dead turtles was again computed via parametric bootstrapping: we randomly sampled the confidence interval for the stranding probability of dead turtles and used the sampled value to obtain the number of dead turtles. Confidence intervals were obtained as described above. Parametric bootstrapping was also used to compute the confidence interval of dead turtles reported by fishers during the interviews: reported numbers of dead turtles were randomly sampled 1,000 times to obtain a corrected 95% confidence interval as described above.
Results
Turtle mortality
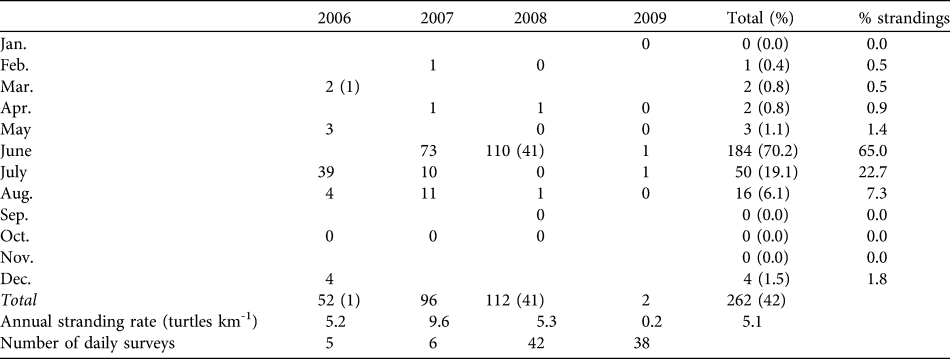
From March 2006 to August 2009 (42 months) we found 262 dead turtles in San Ignacio Lagoon, 261 green turtles and one hawksbill turtle Eretmochelys imbricata: 84%(n = 220) were found stranded on the beach and 16% (n = 42) were observed at sea (Table 1). Mean stranding rate was 5.03 turtles km-1 year-1. Ninety-six percent of total mortality was recorded between May and August (with a peak of 65% of mortality during June). In 2009 the number of dead turtles declined sharply, with only two strandings.
Table 1 Dead turtles observed per month (with number of floating carcasses in parentheses) during 2006–2009. Blank cells indicate the absence of a survey. Stranding rates are calculated based only on stranded carcasses observed.
CCL of dead turtles was 39.7–105.4 cm (n = 211; mean = 59.52 ± SD 11.29; Fig. 2). Using the mean nesting size of 82 cm CCL (Alvarado-Diaz & Figueroa, Reference Alvarado-Diaz and Figueroa1990) we estimated that 94% of the turtles were juveniles. In 84% of the cases external examination of the carcasses did not reveal any apparent cause of mortality and advanced decomposition and scavengers did not allow us to conduct a necropsy. In 16% of the cases death was attributed to fisheries because marks of nets on the flippers and neck of fresh carcasses were present. In the only net we could check we found seven dead turtles entangled. Lateral cuts between carapace and plastron, made by fishers to make the carcass sink, were also observed on several occasions. Necropsies were conducted on 10 fresh carcasses: all presented full stomachs, suggesting that the turtles were healthy and feeding normally, but we found fluid and blood in lungs and net marks on neck and front flippers, suggesting drowning (M.L. Parga, pers. comm.). We tagged 25 carcasses encountered at sea, of which only two stranded on the monitored beach (8%) 1 and 5 days later.
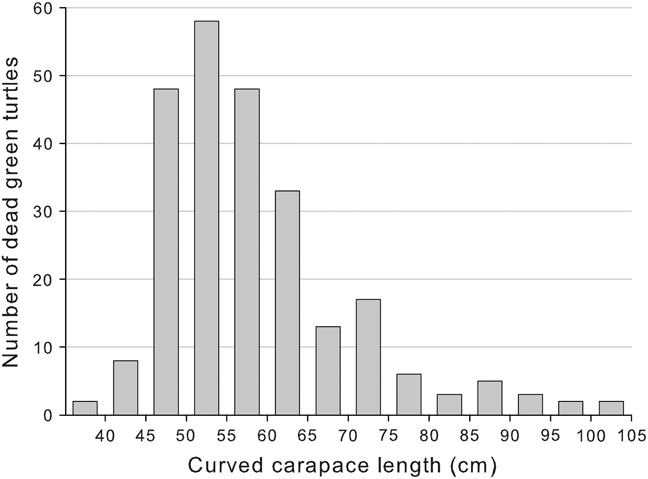
Fig. 2 Size distribution of East Pacific green turtles Chelonia mydas found dead at San Ignacio Lagoon during 2006–2009.
Fishing effort
Nets were present in the lagoon during 15–18 June 2008 and we counted 36 nets in total: 14 nets on day 1, decreasing to two nets on day 4. From day 5 onwards no nets were found within the lagoon as the tides were strong. The length of 26 nets was 138–1,000 m (mean = 415 ± SE 194 m). We estimate that the total net length deployed in the lagoon was 14,951 m, of which 3% (total length 415 m) were found in the upper, 31% (total length 4,659 m) in the middle and 66% (total length 9,877 m) in the lower lagoon (Fig. 3). The kernel density estimator highlighted the presence of at least two high-use areas, a large one in the low region and a small one in the middle region. During daily censuses in June 2009 no nets were observed inside the lagoon.
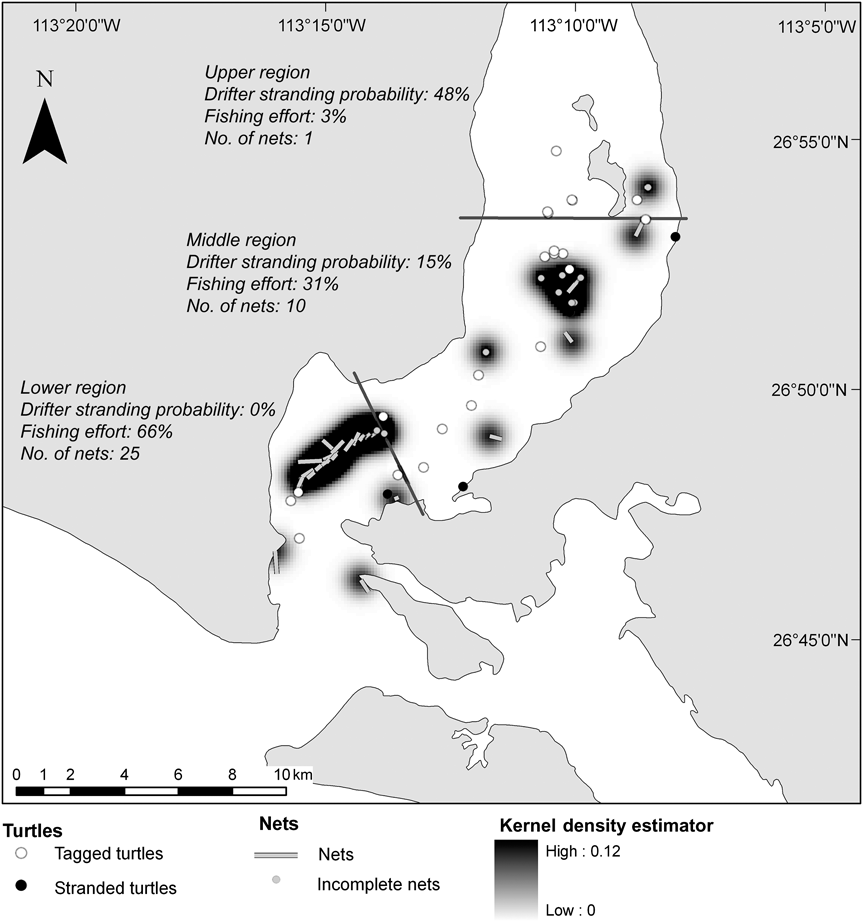
Fig. 3 Location of the gill-nets encountered in San Ignacio Lagoon in June 2008, with the stranding probability of drifters, fishing effort and percentage of nets for the three regions. A kernel density estimator for the distribution of bottom-set mesh-nets identifies the areas of high risk for turtles.
Interviews
Interviewees reported that at least 15 boats per day used gill-nets inside the lagoon during the summer months of 2006–2008. This fishery usually lasted 7–15 days and was considered by all interviewees to be responsible for the high bycatch mortality. They reported an average of seven turtles per day per boat drowned in the nets; one fisher reported the capture of 22 turtles in 1 day. Interviewees reported that guitar-fish catches are high when gravid females enter the lagoon to give birth, and the economic benefits outweigh the risk of getting caught.
Stranding probabilities
Stranding probabilities of drifters decreased, because of the currents, from the upper to lower lagoon (48% in the upper, 15% in the middle and 0% in the lower lagoon; Fig. 3), However, as only two of our marked carcasses stranded we could not include differential stranding probabilities for different parts of the lagoon and had to assume a constant value. The model constructed from the data (Appendix 1) considered only the effect of the stranding probability of drifters as in equation (1) (R2 = 0.59, ![]() = 14.3, P < 0.005) with
= 14.3, P < 0.005) with
Using this model and an estimated ![]() of 0.32 (61 drifters stranded of the 192 released) we predicted a stranding probability for dead turtles of 0.062 (95% confidence interval, CI, 0.035–0.094).
of 0.32 (61 drifters stranded of the 192 released) we predicted a stranding probability for dead turtles of 0.062 (95% confidence interval, CI, 0.035–0.094).
Total mortality estimates
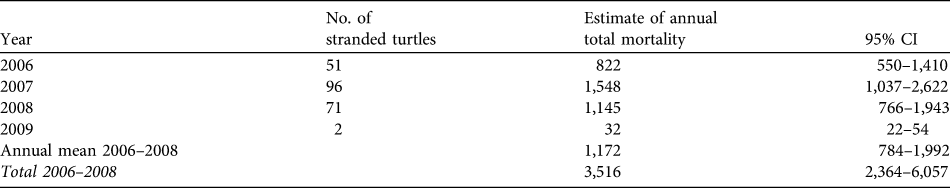
Two hundred and twenty dead turtles were found on the beach during 2006–2009, giving an estimated number of dead turtles (using the stranding probability from the drifters) during the study period of 3,548 (95% CI 2,376–5,976). From 2006–2008, when guitar-fish were caught with set-nets that were left for 24 hours, total mortality was estimated to be 3,516 turtles (95% CI 2,364–6,057) based on our extrapolations, or 1,172 turtles per year. In 2009, when guitar-fish were caught with drift-nets, the average death count dropped sharply to only 32 turtles (95% CI 22–54; Table 2), a reduction of 97%. Bootstrapped data from interviews resulted in a mean mortality of 1,087 turtles per fishing season (95% CI 901–1,286; Appendix 2).
Table 2 Estimates of total turtle mortality from stranding data using a constant stranding probability (P = 0.062, see text for details) estimated from drifters and strandings of carcasses.
Discussion
To our knowledge this study is the first attempt to estimate total bycatch mortality of marine turtles using interviews with fishermen and stranding data independently, combined with estimates of the probability of stranding using data from both marked drifters and carcasses. However, there are some caveats to our methodology: (1) drifter devices are smaller and lighter than turtles, (2) they float all the time instead of initially sinking, as turtles do, until decomposition starts to produce gas, (3) the small number of recovered carcasses did not allow us to estimate a stranding probability as a function of the region where the animal was released. Nevertheless, our methodology will most likely underestimate total mortality and thus our estimates should be considered minimum values. Additionally, we only considered turtles stranded at the monitored beach in our estimate of total mortality in the lagoon. The total length of beach where dead turtles regularly strand is more than twice as long as the section monitored, thus leading to a further underestimate of total mortality.
For any similar future studies of stranding some improvements to our methodology would be beneficial: (1) deploy 20–30 drifters with each floating carcass to compare directly their behaviour and increase the strength of the stranding probability function, (2) monitor floating carcasses and drifters for extensive periods (up to 15 days) during months of high mortality, if possible including VHF or GPS telemetry (Senko et al., Reference Senko, Lopez-Castro, Koch and Nichols2010b), (3) conduct a stranding census twice per day to measure detection rate for both drifters and turtle carcasses, and (4) follow stranded carcasses over extended periods of time to see if they are being moved by scavengers or by tides.
Annual mortality estimates (1,172 turtles per year during 2006–2008) from 220 individuals observed stranded and estimated probabilities of stranding were similar to estimates obtained from interviews with fishers (1,087 turtles per fishing season). As with our estimate from stranded carcasses, the bycatch rate calculated from interviews is probably a minimum estimate. As marine turtles are protected by Mexican law (DOF, 1990) fishers may tend to report lower numbers taken as bycatch to protect themselves and avoid stricter law enforcement in the area. Thus, while the two estimates are close both tend to underestimate total mortality, albeit for different reasons.
Comparing stranding rates can be difficult because of differences in monitoring effort and stranding probability at individual beaches (Peckham et al., Reference Peckham, Maldonado-Diaz, Koch, Mancini, Gaos, Tinker and Nichols2008). However, the annual stranding rate reported for San Ignacio Lagoon (5.81 turtles km-1) is one of the highest reported (Chaloupka et al., Reference Chaloupka, Work, Balazs, Murakawa and Morris2008; Peckham et al., Reference Peckham, Maldonado-Diaz, Koch, Mancini, Gaos, Tinker and Nichols2008). During June 2006–2008, when 65% of the strandings occurred, mean stranding rate rose to 8.49 turtles km-1, comparable to mass strandings in India with 18.6 olive-ridley turtles Lepidochelys olivacea km-1 (Shanker et al., Reference Shanker, Pandav and Choudhury2004), and in Magdalena Bay, Baja California Sur, with 10.8 loggerhead turtles Caretta caretta km-1 (Peckham et al., Reference Peckham, Maldonado-Diaz, Koch, Mancini, Gaos, Tinker and Nichols2008) during summer months.
Even if only 16% of deaths were attributed directly to fisheries the relationship between massive mortality and bycatch is evident and well supported by results from interviews, censuses and observations of the fishery. Eight-inch mesh-nets are banned within lagoons and estuaries by Mexican law (DOF, 2001). However, because of the lack of enforcement some fishers use these nets within the lagoon to catch guitar-fish and halibut. The main cause of turtle mortality in gill-nets is drowning from forced apnea (Casale, Reference Casale2008). Forced submergence of < 10 minutes produces a mortality rate of c. 1% in trawl nets but mortality rate increases to 50–100% for submergence times ≥ 60 minutes (Sasso & Epperly, Reference Sasso and Epperly2006). Nets are set in the morning in the lagoon and checked 24 hours later (R. Mayoral, pers. comm.), resulting in almost 100% mortality for entangled turtles, which cannot surface to breathe as the nets are heavily weighed and anchored on the bottom. The use of drifting nets in 2009 that were checked approximately every 30 minutes explains the drop in bycatch mortality and stranding rates in that year. East Pacific green turtles in San Ignacio Lagoon have a preference for shallow to moderate water depths during the night and for sea-grass areas by day (Senko et al., Reference Senko, Koch, Megill, Carthy, Templeton and Nichols2010a). Furthermore, tidally-oriented movements occur in green turtles in other feeding areas (Brooks et al., Reference Brooks, Harvey and Nichols2009). Sixty-one percent of gill-nets deployed in the lagoon were located in the lower region characterized by moderately deep water, patches of sea-grass and strong tidal currents, suggesting that the risk for turtles of becoming entangled is high in this area.
East Pacific green turtles in San Ignacio Lagoon include individuals from the Revillagigedos breeding stock (Nichols, Reference Nichols2003; P. Dutton, unpubl. data), which is composed of < 100 nesting females each year (NMFS–USFWS, 2007). Recent studies have shown that East Pacific green turtles present high site fidelity, with low connectivity between foraging grounds (Lopez-Castro et al., 2010; Senko et al., Reference Senko, Koch, Megill, Carthy, Templeton and Nichols2010a,b) and spend extended periods in the same area (Seminoff et al., Reference Seminoff, Jones, Resendiz, Nichols and Chaloupka2003). Considering that the illegal guitar-fish fishery has existed in the lagoon for at least 5 years (R. Mayoral, pers. comm.), a mean annual mortality of 1,172 turtles could represent a total mortality of almost 6,000 individuals. Such a figure could have affected the genetic diversity of the East Pacific green turtle, which is fundamental for preserving the species’ resilience to respond to environmental change (Bowen & Karl, Reference Bowen, Karl, Lutz and Musick1997).
In summer 2009 authorities began a weekly patrol and obliged fishers not to anchor their nets and to check them every 30 minutes, resulting in a 97% decline in turtle strandings. However, other studies in the region have also documented high turtle mortality from bycatch and illegal take (Gardner & Nichols, Reference Gardner and Nichols2001; Koch et al., Reference Koch, Nichols, Peckham and de la Toba2006; Peckham et al., Reference Peckham, Diaz, Walli, Ruiz, Crowder and Nichols2007, Reference Peckham, Maldonado-Diaz, Koch, Mancini, Gaos, Tinker and Nichols2008; Mancini & Koch, Reference Mancini and Koch2009) and Nichols (Reference Nichols2003) estimated total mortalities of up to 30,000 turtles per year on the Baja peninsula (direct take for consumption and bycatch). Currently we are compiling the available data from all of north-west Mexico. Preliminary results indicate that the situation with illegal take in the states of Sinaloa and Sonora may be similar to or worse than in Baja California Sur (V. Koch et al., unpubl. data.). There is almost no information available from other parts of Mexico and thus we do not know what impact fisheries have on turtles elsewhere in the country.
San Ignacio Lagoon is emblematic of the potential impact of small-scale fisheries on marine turtle populations. The global death-toll of turtles and other threatened megafauna from unregulated artisanal fisheries in coastal zones is probably high. Ninety-nine percent of 5.1 million fishers globally are employed in small-scale fisheries and 90% of total captures occur in the coastal zone (FAO, 1999). Most of these fisheries are poorly regulated and little is known about bycatch levels and/or illegal take. Further efforts to collect bycatch data from artisanal fleets is clearly needed, as there are several examples showing that their impact on threatened turtles is comparable to, or surpasses, that of industrial fisheries (Godley et al., Reference Godley, Gücü and Broderick1998; Lagueux, Reference Lagueux1998; Koch et al., Reference Koch, Nichols, Peckham and de la Toba2006; Peckham et al., Reference Peckham, Diaz, Walli, Ruiz, Crowder and Nichols2007, Reference Peckham, Maldonado-Diaz, Koch, Mancini, Gaos, Tinker and Nichols2008; Tomás et al., Reference Tomás, Gozalbes, Raga and Godley2008).
The situation in San Ignacio Lagoon has improved substantially. During the 2010 summer fishing season turtle mortality was also exceptionally low because of the presence of enforcement authorities and, consequently, lack of illegal exploitation of guitar-fish. However, information from members of the fishing community suggests that a small group of fishermen would resume their former fishing habits if vigilance should slack, and thus the problem has only been solved temporarily.
Acknowledgements
We thank the Secretaría para el Medio Ambiente y los Recursos Naturales (SEMARNAT) for providing the permits under which this study was conducted (SGPA/DGVS/03846, SGPA/DGVS/03944/07, SGPA/DGVS/03816/08, SGPA/DGVS/05603/09). This study was funded by Consejo Nacional de Ciencias y Tecnologias grant SEMARNAT-2004-C01-277 and by the Earthwatch foundation. We thank all the volunteers, fishers and members of the Grupo Tortuguero who supported our project. AM was supported by Rufford Small Grants for Conservation.
Appendices 1–2
The appendices for this article are available online at http://journals.cambridge.org
Biographical sketches
Agnese Mancini is studying marine turtles in South-East Asia, Pacific Mexico and the Red Sea, with research interests in behavioural ecology and interactions with local fisheries. Volker Koch is studying the population ecology of marine species, energy flow and ecosystem modelling, mollusc aquaculture and marine turtle conservation in Latin America. Jeffrey Seminoff is a member of the IUCN Marine Turtle Specialist Group and is involved in updating marine turtle status assessments for the US Endangered Species Act. His research uses innovative approaches such as stable isotope analyses, bio-telemetry, animal-borne imagery and aerial surveys to elucidate the life history of marine turtles throughout the Pacific Ocean. Bénédicte Madon’s research focuses on new models to estimate animal population size, especially of humpback whale populations. Her interests include ecological statistics, modelling and population management and conservation.







