1. Introduction
GaN has attracted vast interest due to its unique properties and potential applications in optoelectronic and microelectronic devices. However, the dislocation density in GaN heteroepitaxial layers as high as 108 cm −2 Reference Park[1] shortens the lifetime of GaN based devices. The chemical compatibility and lattice/thermal expansion match between AlN and GaN make bulk AlN single crystals potentially suitable for GaN epitaxial growth. In addition, the high thermal conductivity (340W/m·K) and high electrical resistivity make AlN ideal for high power devices Reference Ambacher[2]. Recently, several groups Reference Tanaka, Nakahata, Sogabe, Nakata and Tobioka[3] Reference Liu, Liu, Shi and Edgar[4] Reference Schlesser, Dalmau, Yakimova and Sitar[5] have reported growth of relatively large AlN single crystals, typically at millimeter square scale, produced by the sublimation process, originating from Slack and McNelly’s work in 1970s Reference Slack and McNelly[6]. Understanding the sublimation growth process and the quality of the AlN crystals it produces is key to producing large, low defect density substrates suitable for commercial device fabrication.
AlN has a polar wurtzite structure consisting of closely spaced hexagonal layers, alternating between cation (Al3+) and anion (N3−) layers stacked together along c axis Reference Ambacher[2]. Thus, the basal plane (i.e. the (0001) plane) can be either N- or Al-polarity. The polarity of AlN is important in controlling impurity incorporation and piezoelectric effects in epitaxial GaN films. To date, few studies of the polarity of AlN crystals have been reported. Polarity studies of GaN, however, have been investigated for a long time using many techniques. Hellman Reference Hellman[7] summarized the conclusions of GaN polarity studies from various groups. Wet chemical etching is the most effective and economical method to study the polarity of III-nitrides. Hellman found that the Ga-face of GaN is chemically more stable than the N-face, in that KOH solutions will etch the N-face, but not the Ga-face. In case of AlN, the same rule applied according to Schowalter et al’s observation of etching AlN in KOH solution Reference Schowalter, Rojo, Yakolev, Shusterman, Dovidenko, Wang, Bhat and Slack[8]. Wet chemical etching is also an effective means for defect study. By observing SEM images after etching, one can estimate defect density in the crystal Reference Visconti, Jones, Reshchikov, Cingolani, Morkoç and Molnar[9].
Studying the wet etching of AlN epitaxial films on sapphire substrates in various acids and bases, Pearton et al. Reference Vartuli, Pearton, Lee, Abernathy and Mackenzie[10] concluded that only KOH etches AlN appreciably at room temperature, and the etch rate of AlN films is a strong function of crystal quality. They inferred that annealing improves the quality of heteroepitaxial AlN films, as their etch rate was lower than that of as-deposited films.
In this paper, we report our etching study of AlN single crystals produced by different sublimation growth methods, both self-seeded and seeded on 6H-SiC (0001) substrates.
2. Experimental
We examined several crystals grown in different furnaces and crucible materials. Sample A was prism-shaped needle grown in a graphite heating element furnace using a NbC coated graphite crucible; sample B was a hexagonal platelet grown in the same furnace with a plain graphite crucible; sample C was grown in a microwave-heated furnace; and sample D was grown in a tungsten heating element furnace with a tungsten crucible. Sample A, B, and C employed a self-seeding mechanism, while sample D was a thick AlN film grown directly on a 6H-SiC (Si-face) substrate. Before etching, all samples were cleaned by hydrochloric acid for ten minutes to remove any impurities on the surface. To estimate the appropriate etching time for single crystals, we calculated etch rate of a polycrystalline AlN sample under stirred condition as function of time by measuring the mass and dimension changes due to etching. From this measurement, a standard etching condition for single crystals was set of 10 minutes at 60 °C in 45wt% KOH solution. After etching, all samples were rinsed in 38wt% HCl solution for 5 minutes to neutralize the KOH residues.
3. Results and discussion
The etch rate of polycrystalline AlN as function of time is shown in Figure 1. Within an hour, the etch rate decreased over 70% from 0.55 µm/min to 0.15 µm/min. Since the solution was well stirred, we suspect that the decrease of etch rate was due to the depletion of the easiest etched crystal planes, instead of etchant depletion at the sample surface.

Figure 1. Etch rate of polycrystalline AlN in 45wt% KOH as function of time, at 60 °C.
SEM images of sample A (before and after etching) are shown in Figure 2. Clearly, the planes perpendicular to the basal plane did not etch. Rapid etching was observed on the basal (0001) plane, leading to the formation of the hexagonal hillocks. By analogy to the results reported for GaN Reference Schowalter, Rojo, Yakolev, Shusterman, Dovidenko, Wang, Bhat and Slack[8] Reference Krukowski, Romanowski, Grzegory and Porowski[11], we conclude that this basal plane has a nitrogen polarity. Etching also occurred on the crystal plane inclined at a smaller angle than 90° from the basal plane (Figure 2d). The hillock density for this crystal was approximately 5×107 cm −2.

Figure 2. Sample A before (a) and after (b,c,d) etching. (a) before etching; (b) after etching; (c) enlarged image of circle in (a) after etching; and (d) enlarged image of circle in (b) after etching
Figure 3 displays the etching effects of sample B. Figure 3a shows the AlN crystal before etching; the images 3b and 3c are for 10 minutes etch, and all others are for additional 20 minutes etch. Hexagonal hillocks were observed again on the (0001) basal plane, shown in 3c and 3d. Fig 3e gives us an overview of these hillocks at higher magnification. Hillocks in 3c (10 minutes etching) are approximately 1µm diameters and diameters of hillocks in 3d (additional 20 minutes etch) are about 2µm. Considering the small crystal dimension and self-convection of solution at elevate temperature (60°C), we believe that depletion of etchant was not significant, i.e., the concentration of KOH did not change. Therefore, we conclude that the etch rate decreases with time, as the area of exposed (0001) planes diminishes toward zero. Another interesting observation is that one side of vertically placed (0001) crystal was unchanged by etching (Figure 3f) and is thus most likely of aluminum polarity, while the other side has hexagonal hillocks on it (Figure 3g and 3h) and is hence nitrogen polarity. This confirms that only a single polarity of the (0001) orientation is etched. The hillock density of this crystal was about 109 cm −2.

Figure 3. Sample B before and after etching. (a) before etching; (b) after etching; (c) higher magnification of circle area in (a); (d) after additional 20 minute etch; (e) hillock in (d); (f) one side of vertically placed crystal; (g) the other side of vertical crystal; and (h) higher magnification of (g)
For the crystals grown in the microwave furnace we observed the same etching effect, as shown in Figure 4. By measuring the triangle outlined on Figure 4b, we found that the angle between the basal plane and tilted plane is about 61.6°, which is close to the angle between (0001) and (1

Figure 4. Crystal produced by microwave, after 10 minute etching

Figure 5. AlN crystal grown on SiC substrate for 10 minute etching. (a) before etching; and (b) after etching
4. Conclusions
For self-seeded AlN single crystals, the nitrogen polarity (0001) basal plane initially etched rapidly, while the aluminum polarity basal plane, and prismatic (1
ACKNOWLEDGMENTS
Supports from the Office of Naval Research through grants No. N00014-02-1-0290 and research program sponsored by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Transportation Technologies, as part of the High Temperature Materials Laboratory User Program, Oak Ridge National Laboratory, managed by UT-Battelle, LLC, for the U.S. Department of Energy under contract number DE-AC05-00OR22725 are gratefully appreciated.

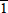 00) planes were not etched. The etch rate of the nitrogen polarity basal plane eventually decreased to zero, as the surface became completely covered with hexagonal hillocks which were bounded by {1
00) planes were not etched. The etch rate of the nitrogen polarity basal plane eventually decreased to zero, as the surface became completely covered with hexagonal hillocks which were bounded by {1




