Cover: Optical microscopy image of TATB after surface layer removal. [M. Taw, J. Yeager, D. Hooks, T. Carvajal, D. Bahr: The mechanical properties of as-grown non-cubic organic molecular crystals assessed by nanoindentation. p. 2728].
Articles
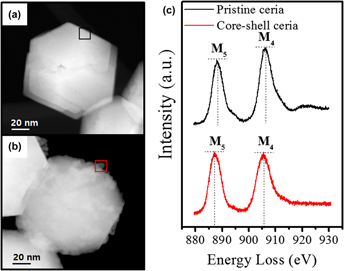
Ce3+-enriched core–shell ceria nanoparticles for silicate adsorption
-
- Published online by Cambridge University Press:
- 27 June 2017, pp. 2829-2836
-
- Article
- Export citation
Front Cover (OFC, IFC) and matter
JMR volume 32 issue 14 Cover and Front matter
-
- Published online by Cambridge University Press:
- 27 July 2017, pp. f1-f5
-
- Article
-
- You have access
- Export citation
Back Cover (OBC, IBC) and matter
JMR volume 32 issue 14 Cover and Back matter
-
- Published online by Cambridge University Press:
- 27 July 2017, pp. b1-b4
-
- Article
-
- You have access
- Export citation