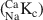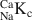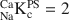1 results
Cation exchange capacity measurements on illite using the sodium and cesium isotope dilution technique: Effects of the index cation, electrolyte concentration and competition: Modeling
-
- Journal:
- Clays and Clay Minerals / Volume 52 / Issue 4 / August 2004
- Published online by Cambridge University Press:
- 01 January 2024, pp. 421-431
-
- Article
-
- You have access
- Export citation