Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Bradbury, M.H.
Baeyens, B.
Geckeis, H.
and
Rabung, Th.
2005.
Sorption of Eu(III)/Cm(III) on Ca-montmorillonite and Na-illite. Part 2: Surface complexation modelling.
Geochimica et Cosmochimica Acta,
Vol. 69,
Issue. 23,
p.
5403.
Rabung, Th.
Pierret, M.C.
Bauer, A.
Geckeis, H.
Bradbury, M.H.
and
Baeyens, B.
2005.
Sorption of Eu(III)/Cm(III) on Ca-montmorillonite and Na-illite. Part 1: Batch sorption and time-resolved laser fluorescence spectroscopy experiments.
Geochimica et Cosmochimica Acta,
Vol. 69,
Issue. 23,
p.
5393.
Tournassat, Christophe
Gailhanou, Hélène
Crouzet, Catherine
Braibant, Gilles
Gautier, Anne
Lassin, Arnault
Blanc, Philippe
and
Gaucher, Eric C.
2007.
Two cation exchange models for direct and inverse modelling of solution major cation composition in equilibrium with illite surfaces.
Geochimica et Cosmochimica Acta,
Vol. 71,
Issue. 5,
p.
1098.
Burzo, E.
2007.
Phyllosilicates.
p.
318.
Timms, W.A.
and
Hendry, M.J.
2007.
Quantifying the impact of cation exchange on long-term solute transport in a clay-rich aquitard.
Journal of Hydrology,
Vol. 332,
Issue. 1-2,
p.
110.
Marques Fernandes, Maria
Baeyens, B.
and
Bradbury, Michael H.
2008.
The influence of carbonate complexation on lanthanide/actinide sorption on montmorillonite.
Radiochimica Acta,
Vol. 96,
Issue. 9-11,
p.
691.
Bradbury, M.H.
and
Baeyens, B.
2009.
Sorption modelling on illite Part I: Titration measurements and the sorption of Ni, Co, Eu and Sn.
Geochimica et Cosmochimica Acta,
Vol. 73,
Issue. 4,
p.
990.
Missana, T.
Alonso, U.
and
García-Gutiérrez, M.
2009.
Experimental study and modelling of selenite sorption onto illite and smectite clays.
Journal of Colloid and Interface Science,
Vol. 334,
Issue. 2,
p.
132.
Bradbury, M.H.
and
Baeyens, B.
2009.
Sorption modelling on illite. Part II: Actinide sorption and linear free energy relationships.
Geochimica et Cosmochimica Acta,
Vol. 73,
Issue. 4,
p.
1004.
Glaus, Martin A.
Frick, Sabrina
Rossé, Roger
and
Loon, Luc R. Van
2010.
Comparative study of tracer diffusion of HTO, 22Na+ and 36Cl− in compacted kaolinite, illite and montmorillonite.
Geochimica et Cosmochimica Acta,
Vol. 74,
Issue. 7,
p.
1999.
Eba, Francois
Aubin Ondo, Jean
Gueu, Soumahoro
Ndong Nlo, Joseph
Kouya Bibo, Raphinos
and
Kouassi Ya, Benjamin
2011.
Treatment of Aqueous Solution of Lead Content by using Natural Mixture of Kaolinite-Albite-Montmorillonite-Illite Clay.
Journal of Applied Sciences,
Vol. 11,
Issue. 14,
p.
2536.
Pearson, F.J.
Tournassat, Christophe
and
Gaucher, Eric C.
2011.
Biogeochemical processes in a clay formation in situ experiment: Part E – Equilibrium controls on chemistry of pore water from the Opalinus Clay, Mont Terri Underground Research Laboratory, Switzerland.
Applied Geochemistry,
Vol. 26,
Issue. 6,
p.
990.
Tremosa, J.
Arcos, D.
Matray, J.M.
Bensenouci, F.
Gaucher, E.C.
Tournassat, C.
and
Hadi, J.
2012.
Geochemical characterization and modelling of the Toarcian/Domerian porewater at the Tournemire underground research laboratory.
Applied Geochemistry,
Vol. 27,
Issue. 7,
p.
1417.
Marques Fernandes, M.
Baeyens, B.
Dähn, R.
Scheinost, A.C.
and
Bradbury, M.H.
2012.
U(VI) sorption on montmorillonite in the absence and presence of carbonate: A macroscopic and microscopic study.
Geochimica et Cosmochimica Acta,
Vol. 93,
Issue. ,
p.
262.
Soltermann, Daniela
Fernandes, Maria Marques
Baeyens, Bart
Dähn, Rainer
Miehé-Brendlé, Jocelyne
Wehrli, Bernhard
and
Bradbury, Michael H.
2013.
Fe(II) Sorption on a Synthetic Montmorillonite. A Combined Macroscopic and Spectroscopic Study.
Environmental Science & Technology,
Vol. 47,
Issue. 13,
p.
6978.
Benedicto, Ana
Begg, James D.
Zhao, Pihong
Kersting, Annie B.
Missana, Tiziana
and
Zavarin, Mavrik
2014.
Effect of major cation water composition on the ion exchange of Np(V) on montmorillonite: NpO2+–Na+–K+–Ca2+–Mg2+ selectivity coefficients.
Applied Geochemistry,
Vol. 47,
Issue. ,
p.
177.
Missana, Tiziana
Benedicto, Ana
García-Gutiérrez, Miguel
and
Alonso, Ursula
2014.
Modeling cesium retention onto Na-, K- and Ca-smectite: Effects of ionic strength, exchange and competing cations on the determination of selectivity coefficients.
Geochimica et Cosmochimica Acta,
Vol. 128,
Issue. ,
p.
266.
Benedicto, Ana
Missana, Tiziana
and
Fernández, Ana María
2014.
Interlayer Collapse Affects on Cesium Adsorption Onto Illite.
Environmental Science & Technology,
Vol. 48,
Issue. 9,
p.
4909.
Mathurin, Frédéric A.
Drake, Henrik
Tullborg, Eva-Lena
Berger, Tobias
Peltola, Pasi
Kalinowski, Birgitta E.
and
Åström, Mats E.
2014.
High cesium concentrations in groundwater in the upper 1.2km of fractured crystalline rock – Influence of groundwater origin and secondary minerals.
Geochimica et Cosmochimica Acta,
Vol. 132,
Issue. ,
p.
187.
Soltermann, Daniela
Marques Fernandes, Maria
Baeyens, Bart
Dähn, Rainer
Joshi, Prachi A.
Scheinost, Andreas C.
and
Gorski, Christopher A.
2014.
Fe(II) Uptake on Natural Montmorillonites. I. Macroscopic and Spectroscopic Characterization.
Environmental Science & Technology,
Vol. 48,
Issue. 15,
p.
8688.
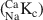 ) were calculated from the experimental data for NaClO4 concentrations from 5.6 × 10−4 to 0.1 M and exhibited a variation from 1.6 to 14.3. A two-site cation exchange model was developed with site capacities and NaCaKc
) were calculated from the experimental data for NaClO4 concentrations from 5.6 × 10−4 to 0.1 M and exhibited a variation from 1.6 to 14.3. A two-site cation exchange model was developed with site capacities and NaCaKc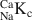 values for each site: planar site capacity =180 meq kg−1, NaCaKcPS=2
values for each site: planar site capacity =180 meq kg−1, NaCaKcPS=2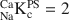 ; type II site capacity = 45 meq kg−1, NaCaKcII=80
; type II site capacity = 45 meq kg−1, NaCaKcII=80 . The model was able to predict the Na and Ca occupancies in the Na-CEC experiments over the whole range of NaClO4 concentrations. It is recommended that Cs should be used instead of Na as the index cation for determining the CEC of illite.
. The model was able to predict the Na and Ca occupancies in the Na-CEC experiments over the whole range of NaClO4 concentrations. It is recommended that Cs should be used instead of Na as the index cation for determining the CEC of illite.

