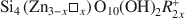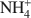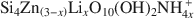Stevensite-like sauconite, with the general composition: \$\end{document}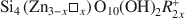 , where □ is a vacant site, was synthesized. The objective was to study the possible migration of some cations (Li+ and Zn2+) within such trioctahedral smectites, under heating, following the so-called ‘Hofmann-Klemen’ (HK) effect. The initial gel was divided into five aliquots and placed in teflon-coated hydrothermal reactors with distilled water, and these were hydrothermally treated at 80, 100, 120, 150, and 200°C, respectively, over 30 days. X-ray diffraction (XRD) analysis confirmed that the samples synthesized were smectites. The number of vacant sites (x) per half unit cell (O10(OH)2) ranged from nearly 0 to 0.23 but no simple relationship was established between x and the temperature of synthesis. The samples were Li+- and Zn2+-saturated, and heated overnight at 300°C (HK treatment). Cation exchange capacity measurements were made by Fourier transform infrared spectroscopy (FTIR) on \$\end{document}
, where □ is a vacant site, was synthesized. The objective was to study the possible migration of some cations (Li+ and Zn2+) within such trioctahedral smectites, under heating, following the so-called ‘Hofmann-Klemen’ (HK) effect. The initial gel was divided into five aliquots and placed in teflon-coated hydrothermal reactors with distilled water, and these were hydrothermally treated at 80, 100, 120, 150, and 200°C, respectively, over 30 days. X-ray diffraction (XRD) analysis confirmed that the samples synthesized were smectites. The number of vacant sites (x) per half unit cell (O10(OH)2) ranged from nearly 0 to 0.23 but no simple relationship was established between x and the temperature of synthesis. The samples were Li+- and Zn2+-saturated, and heated overnight at 300°C (HK treatment). Cation exchange capacity measurements were made by Fourier transform infrared spectroscopy (FTIR) on \$\end{document}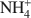 -saturated samples. After LiHK treatment, the structural formula of samples could be expressed as: \$\end{document}
-saturated samples. After LiHK treatment, the structural formula of samples could be expressed as: \$\end{document}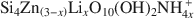 , while after ZnHK treatment, it could be expressed as: Si4Zn3O10(OH)2. Analysis by XRD and FTIR showed that the samples moved from a Zn-stevensite-like structure to Zn-talc-like structure after treatment with ZnHK. These results are interpreted asevidence that Zn2+ (and Li+) migrated into the previously vacant sites under HK treatment.
, while after ZnHK treatment, it could be expressed as: Si4Zn3O10(OH)2. Analysis by XRD and FTIR showed that the samples moved from a Zn-stevensite-like structure to Zn-talc-like structure after treatment with ZnHK. These results are interpreted asevidence that Zn2+ (and Li+) migrated into the previously vacant sites under HK treatment.