Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Van Damme, An
Degryse, Fien
Smolders, Erik
Sarret, Géraldine
Dewit, Julie
Swennen, Rudy
and
Manceau, Alain
2010.
Zinc speciation in mining and smelter contaminated overbank sediments by EXAFS spectroscopy.
Geochimica et Cosmochimica Acta,
Vol. 74,
Issue. 13,
p.
3707.
Madejová, Jana
Pálková, Helena
and
Komadel, Peter
2010.
Spectroscopic Properties of Inorganic and Organometallic Compounds.
p.
22.
Qu, Jin
Cao, Chang-Yan
Hong, You-Li
Chen, Chao-Qiu
Zhu, Pei-Ping
Song, Wei-Guo
and
Wu, Zi-Yu
2012.
New hierarchical zinc silicate nanostructures and their application in lead ion adsorption.
Journal of Materials Chemistry,
Vol. 22,
Issue. 8,
p.
3562.
Petit, S.
and
Madejova, J.
2013.
Handbook of Clay Science.
Vol. 5,
Issue. ,
p.
213.
Mano, Eliana Satiko
Caner, Laurent
Petit, Sabine
Chaves, Arthur Pinto
and
Mexias, André Sampaio
2014.
Mineralogical Characterization of Ni-Bearing Smectites from Niquelândia, Brazil.
Clays and Clay Minerals,
Vol. 62,
Issue. 4,
p.
324.
Decarreau, Alain
Petit, Sabine
Andrieux, Pauline
Villieras, Frederic
Pelletier, Manuel
and
Razafitianamaharavo, Angelina
2014.
Study of Low-Pressure Argon Adsorption on Synthetic Nontronite: Implications for Smectite Crystal Growth.
Clays and Clay Minerals,
Vol. 62,
Issue. 2,
p.
102.
Hildebrando, E. A.
Silva-Valenzuela, M. G.
Neves, R. F.
and
Valenzuela-Diaz, F. R.
2014.
Síntese e caracterização de argila esmectita Zn-estevensita.
Cerâmica,
Vol. 60,
Issue. 354,
p.
273.
Kaufhold, S.
Färber, G.
Dohrmann, R.
Ufer, K.
and
Grathoff, G.
2015.
Zn-rich smectite from the Silver Coin Mine, Nevada, USA.
Clay Minerals,
Vol. 50,
Issue. 4,
p.
417.
2017.
Infrared and Raman Spectroscopies of Clay Minerals.
Vol. 8,
Issue. ,
p.
515.
Zhang, Chaoqun
He, Hongping
Tao, Qi
Ji, Shichao
Li, Shangying
Ma, Lingya
Su, Xiaoli
and
Zhu, Jianxi
2017.
Metal occupancy and its influence on thermal stability of synthetic saponites.
Applied Clay Science,
Vol. 135,
Issue. ,
p.
282.
Madejová, J.
and
Pálková, H.
2017.
Infrared and Raman Spectroscopies of Clay Minerals.
Vol. 8,
Issue. ,
p.
447.
Arfè, Giuseppe
Mondillo, Nicola
Balassone, Giuseppina
Boni, Maria
Cappelletti, Piergiulio
and
Di Palma, Tommaso
2017.
Identification of Zn-Bearing Micas and Clays from the Cristal and Mina Grande Zinc Deposits (Bongará Province, Amazonas Region, Northern Peru).
Minerals,
Vol. 7,
Issue. 11,
p.
214.
Kloprogge, J.T.
2017.
Infrared and Raman Spectroscopies of Clay Minerals.
Vol. 8,
Issue. ,
p.
222.
Balassone, Giuseppina
Nieto, Fernando
Arfè, Giuseppe
Boni, Maria
and
Mondillo, Nicola
2017.
Zn-clay minerals in the Skorpion Zn nonsulfide deposit (Namibia): Identification and genetic clues revealed by HRTEM and AEM study.
Applied Clay Science,
Vol. 150,
Issue. ,
p.
309.
Tosca, Nicholas J.
and
Wright, V. Paul
2018.
Diagenetic pathways linked to labile Mg-clays in lacustrine carbonate reservoirs: a model for the origin of secondary porosity in the Cretaceous pre-salt Barra Velha Formation, offshore Brazil.
Geological Society, London, Special Publications,
Vol. 435,
Issue. 1,
p.
33.
Ebina, Takeo
2018.
Development of Clay‐Based Films.
The Chemical Record,
Vol. 18,
Issue. 7-8,
p.
1020.
Mano, Eliana S.
Caner, Laurent
Petit, Sabine
Chaves, Arthur P.
and
Mexias, André S.
2019.
Ni-smectitic ore behaviour during the Caron process.
Hydrometallurgy,
Vol. 186,
Issue. ,
p.
200.
Zhang, Jing
Zhou, Chun Hui
Petit, Sabine
and
Zhang, Hao
2019.
Hectorite: Synthesis, modification, assembly and applications.
Applied Clay Science,
Vol. 177,
Issue. ,
p.
114.
Dong, Gongyue
Tian, Guangyan
Gong, Linlin
Tang, Qingguo
Li, Mengyao
Meng, Junping
and
Liang, Jinsheng
2020.
Mesoporous zinc silicate composites derived from iron ore tailings for highly efficient dye removal: Structure and morphology evolution.
Microporous and Mesoporous Materials,
Vol. 305,
Issue. ,
p.
110352.
Fonteneau, Lionel
Caner, Laurent
Petit, Sabine
Juillot, Farid
Ploquin, Florian
and
Fritsch, Emmanuel
2020.
Swelling capacity of mixed talc-like/stevensite layers in white/green clay infillings (“deweylite”/ “garnierite”) from serpentine veins of faulted peridotites, New Caledonia.
American Mineralogist,
Vol. 105,
Issue. 10,
p.
1536.
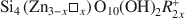 , where □ is a vacant site, was synthesized. The objective was to study the possible migration of some cations (Li+ and Zn2+) within such trioctahedral smectites, under heating, following the so-called ‘Hofmann-Klemen’ (HK) effect. The initial gel was divided into five aliquots and placed in teflon-coated hydrothermal reactors with distilled water, and these were hydrothermally treated at 80, 100, 120, 150, and 200°C, respectively, over 30 days. X-ray diffraction (XRD) analysis confirmed that the samples synthesized were smectites. The number of vacant sites (x) per half unit cell (O10(OH)2) ranged from nearly 0 to 0.23 but no simple relationship was established between x and the temperature of synthesis. The samples were Li+- and Zn2+-saturated, and heated overnight at 300°C (HK treatment). Cation exchange capacity measurements were made by Fourier transform infrared spectroscopy (FTIR) on NH4+\$\end{document}
, where □ is a vacant site, was synthesized. The objective was to study the possible migration of some cations (Li+ and Zn2+) within such trioctahedral smectites, under heating, following the so-called ‘Hofmann-Klemen’ (HK) effect. The initial gel was divided into five aliquots and placed in teflon-coated hydrothermal reactors with distilled water, and these were hydrothermally treated at 80, 100, 120, 150, and 200°C, respectively, over 30 days. X-ray diffraction (XRD) analysis confirmed that the samples synthesized were smectites. The number of vacant sites (x) per half unit cell (O10(OH)2) ranged from nearly 0 to 0.23 but no simple relationship was established between x and the temperature of synthesis. The samples were Li+- and Zn2+-saturated, and heated overnight at 300°C (HK treatment). Cation exchange capacity measurements were made by Fourier transform infrared spectroscopy (FTIR) on NH4+\$\end{document}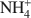 -saturated samples. After LiHK treatment, the structural formula of samples could be expressed as: Si4Zn(3−x)LixO10(OH)2NH4x+\$\end{document}
-saturated samples. After LiHK treatment, the structural formula of samples could be expressed as: Si4Zn(3−x)LixO10(OH)2NH4x+\$\end{document}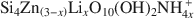 , while after ZnHK treatment, it could be expressed as: Si4Zn3O10(OH)2. Analysis by XRD and FTIR showed that the samples moved from a Zn-stevensite-like structure to Zn-talc-like structure after treatment with ZnHK. These results are interpreted asevidence that Zn2+ (and Li+) migrated into the previously vacant sites under HK treatment.
, while after ZnHK treatment, it could be expressed as: Si4Zn3O10(OH)2. Analysis by XRD and FTIR showed that the samples moved from a Zn-stevensite-like structure to Zn-talc-like structure after treatment with ZnHK. These results are interpreted asevidence that Zn2+ (and Li+) migrated into the previously vacant sites under HK treatment.

