The WHO recommends the use of Fe supplements or home fortificants to improve Fe status and reduce anaemia prevalence among infants and children aged 6–23 months in low-income countries( 1 ). Although home fortificants (such as multiple micronutrient powders (MNP) and small-quantity lipid-based nutrient supplements (LNS)) may have positive effects on children’s micronutrient status, their safety is less well documented, especially in areas where infections are common( Reference De-Regil, Suchdev and Vist 2 ). While some studies suggest that home fortificants are safe( Reference Lemaire, Islam and Shen 3 , Reference Zlotkin, Newton and Aimone 4 ) and may reduce morbidity( Reference Sharieff, Bhutta and Schauer 5 ), others have reported harmful effects. In Pakistan, provision of MNP with or without Zn as home fortificants was associated with increased risk of diarrhoea and reported chest in-drawing in children( Reference Soofi, Cousens and Iqbal 6 ). Findings from a study in Kenya suggest that Fe fortification modifies the gut microbiome, increasing pathogenic bacteria and causing intestinal inflammation( Reference Jaeggi, Kortman and Moretti 7 ). An increased risk of malaria infection and deaths has also been reported in a large trial using Fe and folic acid supplements in Zanzibar, also raising concerns about Fe supplementation in Fe-replete children living in malaria-endemic regions. Excess free Fe circulating in blood may also aid growth of pathogens such as malaria parasites( Reference Sazawal, Black and Ramsan 8 ).
Currently, there is interest in testing the efficacy and effectiveness of providing LNS as home fortificants to prevent undernutrition and micronutrient deficiencies for programmatic use in vulnerable populations. LNS products are similar to MNP because they contain a range of vitamins and minerals, including Fe. There are concerns about Fe provision to children in areas where malaria is endemic( 9 ). However, there is little evidence regarding the safety of Fe-containing LNS. Safety studies have mainly been reported about MNP, although with conflicting results( Reference Lemaire, Islam and Shen 3 , Reference Zlotkin, Newton and Aimone 4 , Reference Soofi, Cousens and Iqbal 6 , Reference Manno, Siame and Larke 10 ). A few reports on LNS provision for prevention of child undernutrition suggest that it is safe( Reference Adu-Afarwuah, Lartey and Brown 11 – Reference Mangani, Ashorn and Maleta 13 ), but the evidence is not conclusive because those studies had either a relatively short duration (i.e. 6 months or less) or insufficient power because of small sample sizes( Reference Dewey, Yang and Boy 14 ).
The iLiNS-DOSE study was a large randomized controlled trial with a primary objective of testing the efficacy of different doses of LNS in promoting linear growth in children in a rural Malawian population. In the main outcome paper, we reported that supplementation with LNS for 1 year did not promote length gain or prevent stunting( Reference Maleta, Phuka and Alho 15 ). However, other studies in Ghana( Reference Adu-Afarwuah, Lartey and Brown 11 ), Burkina Faso( Reference Hess, Abbeddou and Jimenez 16 ) and Malawi( Reference Phuka, Maleta and Thakwalakwa 17 ) reported significant length gain and reduction in stunting associated with LNS. Considering the evidence from these studies, we aimed to assess, as a secondary outcome, the safety of LNS when provided to young children in a malaria-endemic area. In the current analysis, we tested the hypothesis that provision of Fe-containing LNS does not increase infectious disease morbidity when provided to infants and young children for 12 months.
Methods
Study sites and participants
We conducted the study in communities within the catchment areas of Mangochi district hospital and Namwera health centre in the south-eastern part of Malawi. Mangochi district hospital outpatient department serves an estimated population of 100 000 people, whereas Namwera health centre serves a rural population of ~22 000. The hospital catchment area is partly semi-urban, while Namwera is predominantly rural. In this area, the major causes of death among children aged <5 years are malaria (17 %), pneumonia (13 %) and diarrhoeal diseases (11 %)( 18 , 19 ). These diseases are prevalent in the catchment area throughout the year with seasonal fluctuations. The prevalence of HIV is 10·3 % (ages 15–49 years) and an estimated 170 000 children aged 0–14 years are living with HIV/AIDS in Malawi( 20 ).
For a period of 18 months (November 2009 to May 2011), we identified potential participants in the catchment areas through community surveys and invited them to the study clinic for further eligibility assessment. Children who were 5·50 to 6·49 months old, whose guardians had signed informed consent and planned to be available during the whole study period were considered eligible. Exclusion criteria were any severe illness warranting hospital referral, bipedal oedema, Hb<50 g/l, family history of peanut allergy, concurrent participation in another clinical trial and weight-for-height Z-score <−2. We excluded children with weight-for-height Z-score <−2 because these children are at risk of developing severe acute malnutrition. Children with severe acute malnutrition are treated through the national nutritional rehabilitation programmes, therefore were more likely to deviate from our trial protocol. To identify the lowest growth-promoting daily dose and formulation of LNS and to test the hypothesis that milk-free LNS would promote growth equally well as milk-containing LNS, we randomly assigned the children to six groups as follows: milk-containing LNS 10 g/d, 20 g/d and 40 g/d; milk-free LNS 20 g/d and 40 g/d; and a control group which did not receive LNS during the 12-month study period. The nutrient compositions of the five different doses of LNS are reported in Table 1. We used a reduced dose of Fe (6 mg) in the LNS because of safety concerns based on recommendations from the WHO( 9 ). The Zn content (8 mg) was based on the WHO/FAO Recommended Nutrient Intakes for diets with low bioavailability( 21 ). The rationale for selecting specific nutrient levels for LNS is described in detail elsewhere( Reference Arimond, Zeilani and Jungjohann 22 ).
Table 1 Nutrient and energy contents of the food supplements used in the present study

LNS, lipid-based nutrient supplement; RE, retinol equivalents.
Randomization and masking
The details of the randomization process are described in the main outcome paper( Reference Maleta, Phuka and Alho 15 ). Briefly, when the guardian consented to let her infant participate and the infant met all the enrolment criteria, the guardian was asked to choose and open one randomization envelope from a block of six unused envelopes. The envelope contained the participant identification code and supplement code. Randomization into the trial and group allocation was done by a randomizer not participating in the analysis. The investigators involved in data cleaning and analysis were blinded to the group allocation.
Data collection and participant follow-up
Research assistants visited the participants’ homes every week to deliver supplements and collect morbidity data. The data were collected by interviewing the guardians about the child’s health in the previous 7 d using a structured questionnaire. The information was complemented by a picture calendar filled out by the guardians on a daily basis to aid memory of their child’s morbidity status. These were done to minimize problems of recall associated with community morbidity assessments( Reference Arnold, Galiani and Ram 23 ). The research assistants collecting morbidity data knew which children were receiving LNS.
We trained health workers to collect data on non-scheduled visits made to health centres when the child was sick, including data for hospitalizations and hospital deaths. For deaths occurring at home, the information was collected by a verbal autopsy method, previously validated in the study area( Reference Nykanen, Tamaona and Cullinan 24 ). Records of all hospitalizations and deaths were reviewed as serious adverse events (SAE) by a study physician and reported to members of the data and safety monitoring board within 48 h of occurrence.
Anthropometric measurements were taken by research assistants who underwent training and standardization every 6 months. Research assistants who took anthropometric measurements were not aware of group allocation. The details of anthropometric, biochemical and socio-economic measurements and cleaning of anthropometry data are explained in the main outcome paper( Reference Maleta, Phuka and Alho 15 ) and in the statistical analysis plans published on the iLiNS website( 25 ).
Ethics
The study was performed according to International Conference of Harmonization–Good Clinical Practice (ICH-GCP) guidelines and the ethical standards of the Helsinki Declaration. The protocol was reviewed and approved by the Institutional Review Boards of the University of Malawi, College of Medicine (IRB reference number P.01/09/722) and the Pirkanmaa Hospital District, Finland (IRB reference number R09130). At least one guardian signed or thumb-printed an informed consent form before enrolment of each participant. The trial was registered at the clinical trials registry (www.clinicaltrials.gov) with the registration ID of NCT00945698. An independent data safety and monitoring board monitored the incidence of suspected SAE during the trial.
Outcomes
We assessed the following morbidity outcomes at the end of the follow-up: SAE, non-scheduled visits, diagnoses made at non-scheduled visits and guardian-reported morbidity symptoms and disease episodes.
SAE were comprised of hospitalizations and deaths, defined according to the US Department of Health and Human Services Office for Human Research Protections( 26 ). Non-scheduled visits were defined as visits made by the participants to any health facility because of illness. At each non-scheduled visit, a diagnosis of malaria, gastroenteritis, acute respiratory infection or other illnesses was made by health workers.
For home visits, diagnoses of gastroenteritis, acute respiratory infection and ‘undefined fever’ were derived from a combination of guardian-reported morbidity symptoms recorded on one or more days.
To ensure that the diagnoses derived from symptoms at home visits were mutually exclusive a diagnosis algorithm was created, whereby any diarrhoea episode (three or more loose stools in 24 h) was categorized as gastroenteritis with or without other symptoms. If diarrhoea was absent but there was presence of any respiratory symptoms (cough, rapid or difficult breathing and nasal discharge) with or without fever, a diagnosis of acute respiratory infection was made. Fever episodes in the absence of diarrhoea and respiratory symptoms, with or without other symptoms, were categorized as ‘undefined fever’. The rest of the symptoms in the absence of diarrhoea, respiratory symptoms and fever were categorized as other illnesses. For all diseases, an episode was defined as the period starting from the day the child had symptoms if preceded by at least two days of either no symptoms or no data. The episode ended on the last day the child had symptoms which was then followed by at least two symptom-free days.
We defined anaemia as Hb<105 g/l based on suggested reference values for infants( Reference Domellof, Dewey and Lonnerdal 27 ). We defined Fe deficiency as Zn protoporphyrin>70 µmol/mol haem, measured from washed red blood cells( Reference Hastka, Lasserre and Schwarzbeck 28 , Reference Zimmermann, Molinari and Staubli-Asobayire 29 ).
Sample size calculation
The sample size was calculated based on the primary objective of the main study: to test the hypothesis of non-inferiority of LNS without milk on change in length-for-age Z-score as compared with LNS containing milk. Assuming an sd for the change in length-for-age Z-score of 1·0, a predetermined non-inferiority margin of 0·25 Z-score units and an estimated 15 % attrition rate, a sample size of 320 per group was estimated to provide the trial with 90 % power and 95 % confidence (one-sided test) to discard an inferiority null hypothesis. Morbidity (the outcome for the present paper) was a secondary outcome of the study. We did not calculate a separate sample size or post hoc power for the morbidity outcome. We relied on the confidence interval to determine if the sample size was adequate for each of the morbidity outcomes, as recommended by several scholars( Reference Levine and Ensom 30 , Reference Feinstein and Concato 31 ).
Data entry and management
The data were double-entered using Microsoft® Access, REDCap™ and TELEform ®. Typographical errors, extreme observations and discrepancies were resolved prior to breaking the randomization code. Analyses were done using the statistical software package Stata version 12·1.
Statistical analysis
Our hypothesis was that the risk of morbidity among children would not be significantly higher in the intervention groups compared with the control group. We used a non-inferiority approach to compare the risk of morbidity between the intervention groups and the control group. We chose a predefined non-inferiority margin of no greater than 20 % increase in morbidity in the intervention groups compared with the control group to conclude that there was no difference in morbidity. We assumed that an increase in morbidity of 20 % or more in the LNS groups relative to the control would be clinically significant, with negative impact on the overall health of the children. There is no agreed definition of non-inferiority in this context, but an increase between 15 % and 20 % in morbidity has previously been considered clinically significant( Reference Lemaire, Islam and Shen 3 , Reference Zlotkin, Newton and Aimone 4 , Reference Soofi, Cousens and Iqbal 6 , Reference Sazawal, Black and Ramsan 8 , Reference Mangani, Ashorn and Maleta 13 ). The non-inferiority approach and the margin of 20 % were predefined in the statistical analysis plan published at the iLiNS website( 25 ) before starting the analysis.
We intended to confirm non-inferiority if the entire 95 % CI (two-sided) for the risk ratio (RR)/incidence rate ratio (IRR) was below the value of 1·20. For each morbidity outcome, we anticipated the following four possible conclusions: (i) if the upper bound of the 95 % CI for the RR/IRR was <1·20, a conclusion of non-inferiority would be made; (ii) if the lower bound of the 95 % CI for the RR/IRR was >1·20, a conclusion of inferiority (suggesting a harmful effect) would be made; (iii) if the upper bound of the 95 % CI for the RR/IRR was <1·00, a conclusion of superiority (suggesting a beneficial effect) would be made; and (iv) If the upper bound of the 95 % CI for the RR/IRR was >1·20 and lower bound was <1·20, the findings would be considered inconclusive for target group inference.
Risks of experiencing an SAE (hospitalization or death) were calculated as numbers of participants experiencing an SAE divided by the total number of participants in each group. We used generalized linear modelling (log-binomial family) to estimate and compare the risks between the intervention groups and the control group, reported as RR (95 % CI). Longitudinal prevalences of common morbidity symptoms were defined as proportions of days when the child had an illness among all days of observation for each child. We compared the longitudinal prevalence for each intervention group with the control group by first log-transforming the data, then performed ordinary least squares regression, and exponentiated the regression coefficients and their 95 % CI. Incidences of non-scheduled visits and guardian-reported disease episodes in each group were calculated as the sum of visits or episodes across individuals divided by total child-years in each group. We used negative binomial regression to compare the incidences between the intervention groups and the control group, reported as IRR (95 % CI).
The primary analysis was done on the intention-to-treat basis. As secondary analysis, the outcomes were fitted in models that included the following covariates at baseline: age, sex, weight-for-length Z-score, weight-for-age Z-score, length-for-age Z-score, Fe status, Hb status, seasonality, maternal education, marital status, household food insecurity (using the Household Food Insecurity Access Scale), household water source and sanitation.
We planned first to compare between the milk and non-milk groups of the same dose, then proceed to collapse the groups if there were no differences between the milk and non-milk groups of the same LNS dose. We outlined this plan in the statistical analysis plan published at our website( 25 ). Although the Fe content was originally the same in all LNS doses, we observed differences in daily intake of LNS between the groups (those given the higher doses of LNS/d consumed a lower proportion)( Reference Hemsworth, Kumwenda and Rehman 32 ), suggesting that the actual daily dose of Fe and other micronutrients also varied among the intervention groups. Therefore we analysed the groups according to the different daily doses of LNS provided.
Results
We conducted the iLiNS-DOSE study between November 2009 and May 2012. Out of 2136 infants who came to the study clinic for assessment, 1932 were enrolled and randomized into the six study groups. The group allocation, reasons for exclusion and loss to follow-up are shown in Fig. 1. At baseline, the mean age of the participants was 5·9 (sd 0·3) months and their mean length-for-age Z-score and weight-for-height Z-score were −1·4 (sd 1·1) and +0·3 (sd 1·1), respectively. The proportions of children with stunting, a positive malaria test, severe anaemia (Hb<80 g/l), moderate to severe anaemia (Hb<105 g/l), Fe deficiency (Zn protoporphyrin>70 µmol/mol haem) or Fe-deficiency anaemia were 29·3 %, 16·3 %, 7·9 %, 50·8 %, 66·0 % and 42·0 %, respectively. The reported bed-net utilization by participants was 81·0 %. There were no differences between the groups in the baseline characteristics (Table 2). No episodes of suspected allergy or intolerance of the supplement were recorded during the trial.
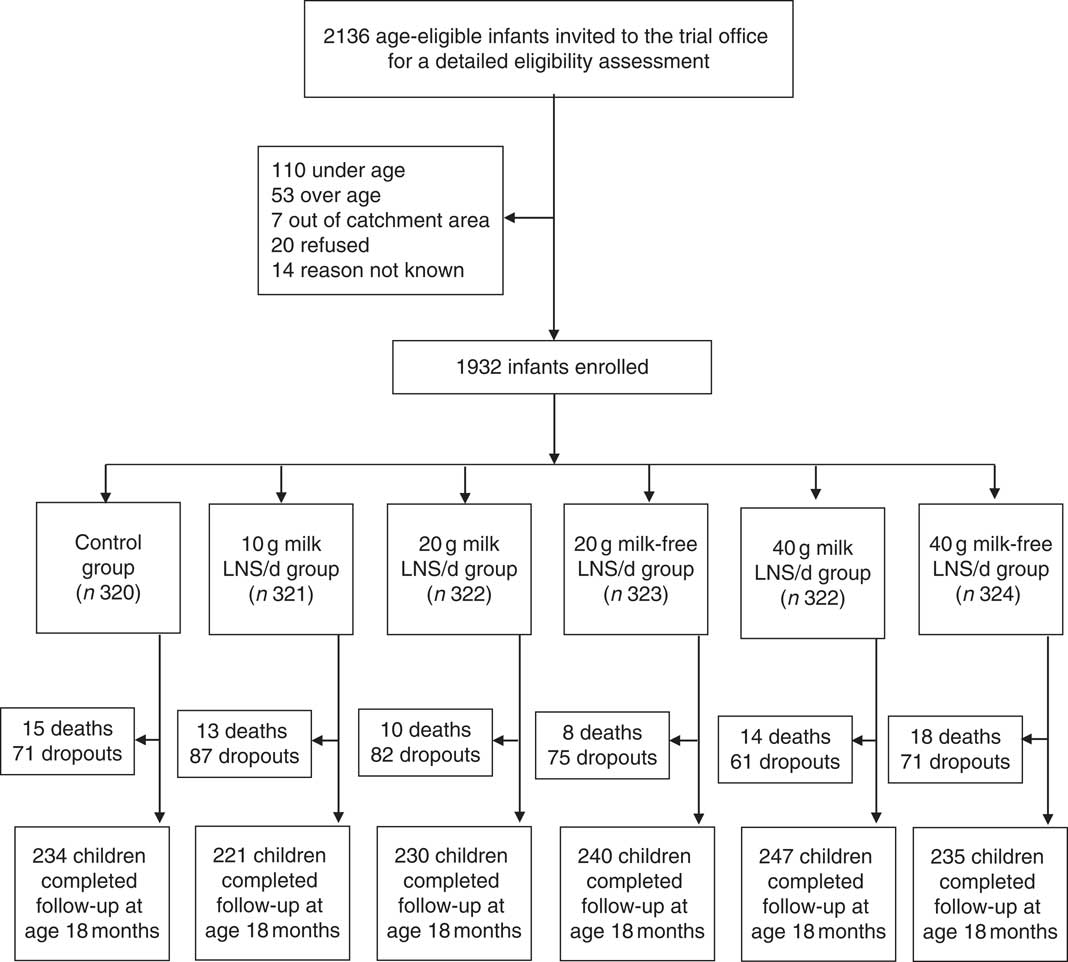
Fig. 1 Participant flow in the present study (LNS, lipid-based nutrient supplement)
Table 2 Baseline characteristics of the participating rural Malawian infants and young children, iLiNS-DOSE study, November 2009–May 2012
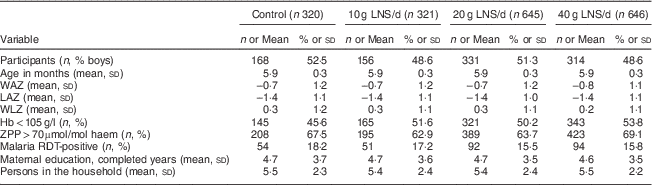
LNS, lipid-based nutrient supplement; WAZ, weight-for-age Z-score; LAZ, length-for-age Z-score; WLZ, weight-for-length Z-score; ZPP, Zn protoporphyrin; RDT, rapid diagnostic test.
The enrolled children contributed a total of 1306 child-years of follow-up, i.e. the mean length of follow-up was 251 (sd 94) d/child. A total of 1534 (79·4 %) children remained in follow-up at 18 months of age and we managed to see 1407 (72·8 %) children on the final home visit. There were no intergroup differences either in the mean length of follow-up (P=0·974) or the proportion of children who remained in the study until its end (P=0·334). We did not see differences in morbidity outcomes between the milk and non-milk groups of the same dose (each P>0·05); therefore we proceeded to collapse the groups. We present all the results by daily ration of LNS (0, 10, 20 or 40 g/d), irrespective of the milk content of the supplement.
We recorded 271 SAE among the study participants. Of these, seventy-eight were deaths (4·0 % of the enrolled participants) and 193 were hospitalizations. Compared with the control group, the 95 % CI for the RR of experiencing an SAE was entirely below 1·20 (suggestive of non-inferiority) in the 10 g LNS/d group, entirely below 1·00 (suggestive of a protective effect) in the 20 g LNS/d group and ranged from 0·66 to 1·25 (inconclusive) in the 40 g LNS/d group. The 95 % CI for the RR of hospitalizations was entirely below 1·20 (suggestive of non-inferiority) in the 10 and 20 g LNS/d groups, and ranged from 0·57 to 1·22 (inconclusive) in the 40 g LNS/d group (Table 3). The 95 % CI for the risk of death was entirely below 1·20 (suggestive of non-inferiority) in the 20 g LNS/d group, and ranged from 0·42 to 1·79 and 0·58 to 1·92 (inconclusive) in the 10 and 40 g LNS/d groups, respectively (Table 3).
Table 3 Risk of serious adverse events (SAE) by intervention group among rural Malawian infants and young children, iLiNS-DOSE study, November 2009–May 2012
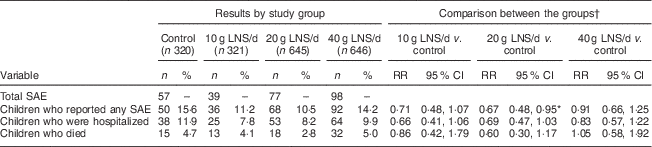
LNS, lipid-based nutrient supplement; RR, risk ratio.
* Statistically significant result.
† Groups were compared using generalized linear modelling (log-binomial family).
In total, we recorded 9034 non-scheduled visits to health facilities due to illnesses (5·2 non-scheduled visits/child per year of follow-up). Compared with the control group, the 95 % CI for the IRR of non-scheduled visits was entirely below 1·20 (suggestive of non-inferiority) in the 10 and 20 g LNS/d groups. The incidence was 13 % higher in the 40 g LNS/d group but the lower bound of the 95 % CI for the IRR was <1·20 (inconclusive; Table 4). For non-scheduled visits due to malaria (confirmed by rapid diagnostic test), acute respiratory infection and other illnesses, the incidences were, respectively, 21 %, 14 % and 13 % higher in the 40 g LNS/d group than in the control group, but the comparisons were also inconclusive (Table 4).
Table 4 IncidenceFootnote † of non-scheduled visits and clinical diagnoses among rural Malawian infants and young children, iLiNS-DOSE study, November 2009–May 2012

LNS, lipid-based nutrient supplement; IRR, incidence rate ratio; ARI, acute respiratory infection.
* Statistically significant results.
† Incidence=number of cases/child per year of follow-up.
‡ Groups were compared using negative binomial regression.
§ Measured by rapid diagnostic test (RDT) for malaria parasites based on histidine-rich protein (HRP-II).
|| Other diseases=sepsis, measles, anaemia, etc.
The mean longitudinal prevalence of all guardian-reported illness symptoms was 29·1 (sd 19·6) %. Compared with the control group, the 95 % CI for the geometric means ratio of the longitudinal prevalence of all symptoms was entirely below 1·20 (suggestive of non-inferiority) in the 10 g LNS/d and 40 g LNS/d groups and ranged from 0·97 to 1·22 (inconclusive) in the 20 g LNS/d group (Table 5). For most of the individual symptoms, comparisons of the longitudinal prevalences between the LNS groups and the control group were inconclusive (Table 5).
Table 5 Longitudinal prevalenceFootnote † of guardian-reported common childhood morbidity symptoms among rural Malawian infants and young children, iLiNS-DOSE study, November 2009–May 2012
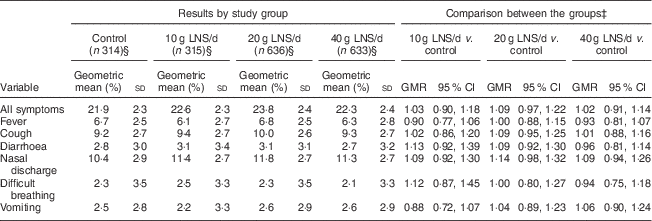
LNS, lipid-based nutrient supplement; GMR, geometric means ratio.
† Longitudinal prevalence = number of days with symptom/total number of days of follow-up.
‡ Groups were compared using ordinary least squares regression.
§ Excluded thirty-four participants from analysis due to missing data (six in the control group, six in the 10 g LNS/d group, nine in the 20 g LNS/d group and thirteen in the 40 g LNS/d group).
From the guardians’ symptom recalls, we identified 19 690 separate illness episodes among the study participants. We excluded thirty-four children from this analysis because of missing data. The mean incidence of illness episodes was 15·6 (sd 8·8)/child per follow-up year. Compared with the control group, the 95 % CI for the IRR of all illnesses, ‘undefined fever’ and acute respiratory infection episodes were entirely below 1·20 (suggestive of non-inferiority) in all the intervention groups (Table 6).
Table 6 IncidenceFootnote † of guardian-reported disease episodes among rural Malawian infants and young children, iLiNS-DOSE study, November 2009–May 2012

LNS, lipid-based nutrient supplement; IRR, incidence rate ratio; ARI, acute respiratory infection.
† Incidence=number of episodes/child per year of follow up.
‡ Groups were compared using negative binomial regression.
Finally, we combined the intervention groups into one group to compare all participants who received LNS with those who did not. In this comparison, the 95 % CI for all morbidity outcomes were entirely below 1·20 (suggestive of non-inferiority); the 95 % CI for the incidence of non-scheduled visits was just touching the non-inferiority margin of 1·20. The 95 % CI for the risk of death was too wide to draw any conclusions (Fig. 2). Adjusting for baseline anaemia and Fe status did not significantly alter any of the results (data not shown).
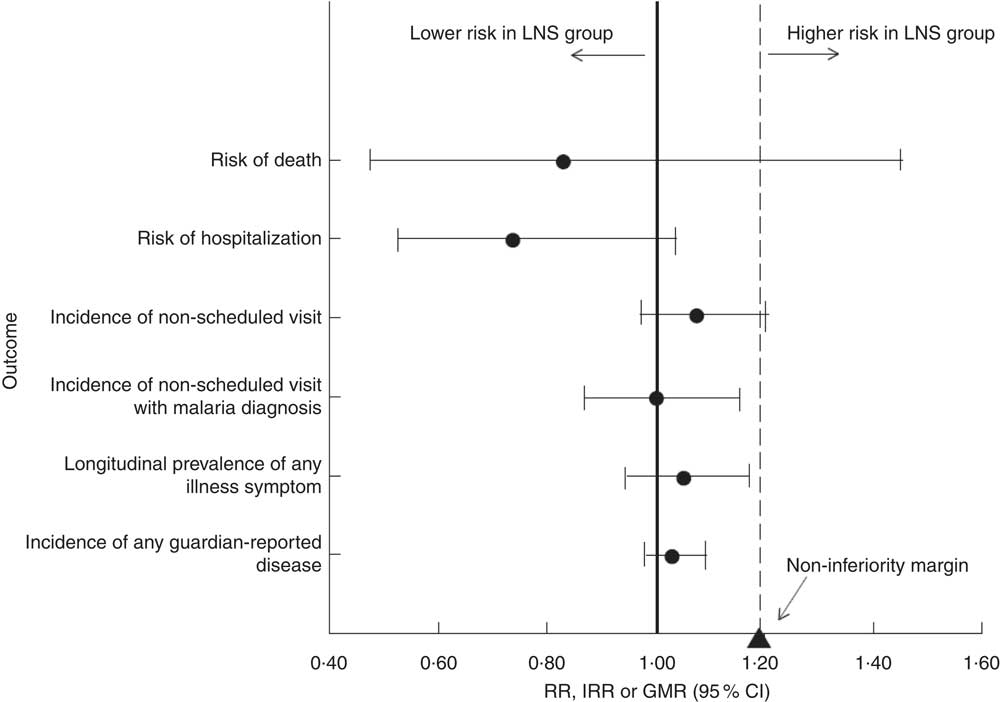
Fig. 2 Plot of selected morbidity outcomes among rural Malawian infants and young children, iLiNS-DOSE study, November 2009–May 2012. All intervention groups receiving lipid-based nutrient supplement (LNS) were pooled together and compared with the control group, with results (●) reported as incidence rate ratios (IRR), relative risks (RR) or geometric means ratios (GMR) with their 95 % CI (represented by horizontal lines). The vertical dashed line represents the margin of non-inferiority. The solid line represents the null effect. None of the point estimates and their 95 % CI fell entirely above the non-inferiority margin (1·20; ▲). We conclude that LNS is does not increase morbidity
Discussion
We tested the hypothesis that long-term supplementation with LNS containing 6 mg Fe would not increase morbidity in children. In a sample of 1932 Malawian infants and young children aged 6–18 months, we found that daily provision of LNS for 1 year was not associated with excess hospitalizations, episodes of common childhood diseases or symptoms when all intervention groups were combined. The incidence of non-scheduled visits was inconclusive. For group comparisons, the 10 and 20 g LNS/d groups had similar outcomes to the control group, except the risk of SAE which was significantly lower in the 20 g LNS/d group. For the 40 g LNS/d group, the comparisons were inconclusive, with increased incidence of non-scheduled visits due to malaria in this LNS group. Our study was not powered to assess mortality outcome.
Our study had several strengths: random allocation of participants; blinding of the investigators involved in data analysis; frequent morbidity data collection; and the year-long follow-up period. Our findings may need to be interpreted with caution since the primary outcome results showed no effect of LNS on growth or stunting( Reference Maleta, Phuka and Alho 15 ). However, studies prior to this reported significant length gain and reduction in stunting associated with LNS( Reference Adu-Afarwuah, Lartey and Brown 11 , Reference Hess, Abbeddou and Jimenez 16 , Reference Phuka, Maleta and Thakwalakwa 17 ). Therefore we believe our results showing that LNS is safe are relevant on a wider scale.
Potential causes of bias were: higher than anticipated attrition (20·4 %); diagnostic malaria tests not done at home visits; and possible differential reporting of illness by guardians who were not blinded. Theoretically, it is also possible that the research assistants may have under-reported morbidity for children in the LNS groups since they were not blinded. However, we had other objective tools for morbidity data collection such as non-scheduled visits reported by clinicians not employed by the study and SAE assessed by the study physician who was blinded to group allocation. All these showed consistent results of no difference in morbidity, except at non-scheduled visits where malaria-related visits were inconclusive in the 40 g LNS/d group. There were no intergroup differences in proportion of children whose data were available and all participants were included in the analysis up to the time of their dropout or death, suggesting that attrition was balanced among the groups. Reimbursement of medical costs could also inflate the non-scheduled visits data. However, Malawi provides a free national health-care system in public health facilities where most of our participants were treated, and we did not reimburse transport for non-scheduled visits. A study in children aged 6–36 months in Chad( Reference Huybregts, Houngbe and Salpeteur 12 ) recorded an incidence of reported disease episodes of 1·17 per child-month (or 14·04 per child-year) which is close to our findings, suggesting that the high disease incidence at home visits was a true observation. Thus, we believe our results are still valid and representative of the target population, suggesting that 10 and 20 g LNS/d did not increase morbidity in our population.
Our findings of no excess morbidity in the 10 and 20 g LNS/d groups are similar to those of a previous study done in Malawi, but in a different area. In that study of 840 children, daily provision of 54 g LNS (containing the same amount of Fe as the LNS used in the present study, 6 mg) did not increase malaria or respiratory morbidity( Reference Mangani, Ashorn and Maleta 13 ). Similar findings have been reported in previous studies that used LNS( Reference Adu-Afarwuah, Lartey and Brown 11 , Reference Huybregts, Houngbe and Salpeteur 12 ) or MNP( Reference Lemaire, Islam and Shen 3 – Reference Sharieff, Bhutta and Schauer 5 ). In our study, although we did not routinely test for malaria during home visits, cases of ‘undefined fever’ could be classified as suspected malaria cases according to the Integrated Management of Childhood Illnesses classification for high-malaria-risk areas( 33 ). In general, Fe-containing home fortificants are assumed to be safer than Fe supplements (such as liquid Fe drops) for children living in areas where infectious diseases are common because home fortificants provide a physiological dose of Fe distributed throughout the day, which potentially avoids the adverse effects associated with Fe given as a bolus dose( Reference Maleta, Phuka and Alho 15 ). However, another key mechanism by which Fe may mediate malaria morbidity is by increasing reticulocytosis( Reference Clark, Goheen and Fulford 34 ). In unadjusted and preliminary analyses of Hb and Zn protoporphyrin status in this study cohort, LNS provision was associated with improved Fe status and a reduction in the prevalence of Fe deficiency, but no improvement in blood Hb concentration. Thus, the Fe in the LNS improved Fe stores but may not have stimulated reticulocytosis, which might explain the lack of adverse effects on malaria morbidity.
Our findings are different from the reports of the studies in Pakistan( Reference Soofi, Cousens and Iqbal 6 ), Kenya( Reference Jaeggi, Kortman and Moretti 7 ), Pemba( Reference Sazawal, Black and Ramsan 8 ), Zambia( Reference Manno, Siame and Larke 10 ) and Cote d’Ivoire( Reference Zimmermann, Chassard and Rohner 35 ). These studies reported increases in malaria-related hospitalizations and deaths, respiratory infections, diarrhoea and intestinal inflammation associated with Fe supplements or Fe-containing MNP provision in children.
These differences could be due to: (i) the dose of Fe; (ii) prevalence of Fe deficiency in the study population; and (iii) intensive morbidity surveillance.
The dose of Fe used in the above studies was 12·5 mg/d, whereas we used 6 mg/d in our study. We advised that the LNS be eaten on two or more occasions during the day, so as to limit the amount of Fe ingested in a single meal( Reference Arimond, Zeilani and Jungjohann 22 ). This might have eliminated the detrimental effects of Fe. The high prevalence of Fe deficiency (66 %) in our population may also offer protection to infections aggravated by provision of Fe as suggested by other studies in Africa( Reference Jonker, Calis and van Hensbroek 36 , Reference Nyakeriga, Troye-Blomberg and Dorfman 37 ), although not all( Reference Veenemans, Milligan and Prentice 38 ). In the Ghana and Pemba studies, children with Fe deficiency at baseline were not adversely affected by the intervention( Reference Zlotkin, Newton and Aimone 4 , Reference Sazawal, Black and Ramsan 8 ). We provided intensive morbidity surveillance and referral for treatment to the national health system which may have improved the overall health of the children, an observation also highlighted in the Ghana and Pemba studies( Reference Zlotkin, Newton and Aimone 4 , Reference Sazawal, Black and Ramsan 8 ). Thus, it appears that although in some settings provision of Fe may increase the risk of morbidity, this was not the case in our population with a high prevalence of Fe deficiency, using a modest dose of Fe in LNS and providing morbidity surveillance.
The finding that the 40 g LNS/d dose may have been associated with excess malaria-related non-scheduled visits is puzzling, especially given that the 10 and 20 g LNS/d doses were conclusively not associated with increased morbidity. We do not have a clear biological explanation for this because the dose of Fe was intended to be similar in all the LNS groups. Our dietary intake data suggest that children in the 40 g LNS/d group were actually getting less Fe, as the proportion of LNS eaten by children in this group was only about half that of the 10 g LNS/d group( Reference Hemsworth, Kumwenda and Rehman 32 ). In addition, there were no differences in breast milk intake observed between the groups in intention-to-treat analysis( Reference Kumwenda, Dewey and Hemsworth 39 ). Other factors such as modifications of gut microbiota( Reference Jaeggi, Kortman and Moretti 7 , Reference Gordon, Dewey and Mills 40 ) may be involved in the pathways that link home fortificants and infections, and these require further study.
Conclusion
In conclusion, long-term provision of 10 and 20 g LNS/d containing 6 mg Fe/d did not increase morbidity in infants and young children in a population where Fe deficiency and infectious diseases are both common. Provision of 40 g LNS/d did not affect guardian-reported illness symptoms and episodes but may have increased non-scheduled visits because of malaria. A larger study would be needed to assess the effect of LNS on child mortality.
Acknowledgements
Acknowledgements: The authors thank the study participants, the local communities, the health service staff and their research personnel at the study sites as well as members of the trial’s data safety and monitoring board, the iLiNS extended research team and the iLiNS-Project Steering Committee (http://www.ilins.org) for their contributions in all stages of the study. Financial support: This publication is funded by a grant to the University of California, Davis from the Bill & Melinda Gates Foundation. The findings and conclusions contained within the article are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Conflict of interest: The authors declare no conflict of interest. Authorship: The authors’ responsibilities were as follows: K.M., J.P., K.G.D., S.A.V., Y.B.C., U.A. and P.A. designed the study; J.B., L.A., K.M., J.P., S.A.V., Y.B.C., U.A. and P.A. conducted the study; J.B. analysed data and wrote the paper, with critical input and comments from all other authors; J.B. and P.A. had primary responsibility for final content. All authors read and approved the final manuscript. Ethics of human subject participation: The study was performed according to International Conference of Harmonization–Good Clinical Practice (ICH-GCP) guidelines and the ethical standards of the Helsinki Declaration. The protocol was reviewed and approved by the Institutional Review Boards of the University of Malawi, College of Medicine (IRB reference number P.01/09/722) and the Pirkanmaa Hospital District, Finland (IRB reference number R09130). At least one guardian signed or thumb-printed an informed consent form before enrolment of each participant. The trial was registered at the clinical trials registry (www.clinicaltrials.gov) with the registration ID of NCT00945698. An independent data safety and monitoring board monitored the incidence of suspected SAE during the trial.











