Methionine (Met) is the first limiting amino acid in chicken diets. Dietary Met deficiency has been demonstrated to impair chicken growth( Reference Corzo, Kidd and Dozier 1 ); thus, it is important to have accurate information on Met requirement of chicks for formulating diets to optimise their growth and production. The requirement of Met for growth and maintenance would be expected to vary with factors that influence maximum growth and feed intake( Reference Chamruspollert, Pesti and Bakalli 2 ). Extensive work has been carried out to estimate the Met requirement of broilers under various conditions such as sex, dietary nutrients and rearing environment( Reference Chamruspollert, Pesti and Bakalli 2 – Reference Chamruspollert, Pesti and Bakalli 4 ). However, none of such studies has taken the hatching weight (HW) of broiler chicks into consideration. In fact, the performance of broiler chicks is largely influenced by HW( Reference Leandro, Cunha and Stringhini 5 – Reference Mendes, Paixão and Restelatto 7 ). The average HW may vary largely from 36 to 48 g, depending on egg weight and hatching process( Reference Shalev and Pasternak 8 ). Sklan et al. ( Reference Sklan, Heifetz and Halevy 9 ) reported that the marketing weight was about 1·1-fold higher in broilers hatching at 53·1 (sem 0·5) g than in those hatching at 43·5 (sem 0·5) g and suggested that this growth process was regulated by skeletal muscle growth. It has been proven that muscle growth is stimulated by the insulin-like growth factor-I (IGF-I) signalling pathway( Reference McMurtry 10 , Reference Beccavin, Chevalier and Cogburn 11 ), which is activated by amino acids, especially Met( Reference Dozier, Kidd and Corzo 12 ). Met deficiency has been shown to result in lower breast muscle weight in broilers( Reference Corzo, Kidd and Dozier 1 ), and a positive effect of increasing dietary Met levels on chicken breast muscle yield has also been reported( Reference Hickling, Guenter and Jackson 13 ). Whether high-Met diets can improve the performance and muscle growth of broilers with lower HW is unknown. However, to our knowledge, the responses of chicks with different HW to dietary Met have not been reported.
Skeletal muscle hypertrophy in response to IGF-I is critically mediated by the serine/threonine kinase Akt, the downstream targets of which include target of rapamycin (TOR), eIF4E-binding protein 1 (4EBP1) and ribosomal protein S6 kinase 1 (S6K1), key regulators involved in mRNA translation and protein synthesis( Reference Bodine, Stitt and Gonzalez 14 ). IGF-I has also been shown to prevent the expression of muscle atrophy-induced ubiquitin ligases, atrogin-1 and muscle ring finger-1 (MuRF1), by inhibiting the forkhead box O (FOXO) subfamily of transcription factors( Reference Stitt, Drujan and Clarke 15 , Reference Latres, Amini and Amini 16 ), which consists of four members, FOXO1, FOXO3, FOXO4 and FOXO6( Reference Burgering 17 ). Daily variations in dietary lysine content alter TOR and FOXO phosphorylation and atrogin-1 mRNA expression in chicken pectoralis major muscle( Reference Tesseraud, Bouvarel and Collin 18 ). However, little information is available on the response of these pathways to dietary Met in chickens.
The objective of the present study was to evaluate the effects of dietary Met on the performance, breast muscle growth and expression of genes associated with the IGF-I signalling pathway in broilers with different HW.
Materials and methods
Bird husbandry, diets and experimental design
All experimental procedures involving animals were approved by the Nanjing Agricultural University Institutional Animal Care and Use Committee.
A 42 d feeding trial was conducted with 192 1-d-old Arbor Acres broiler chicks with different HW (heavy: 48·3 (sem 0·1) g and light: 41·7 (sem 0·1) g) from the same maternal flock (47 weeks of age). They were allocated to a randomised block design with a 2 (HW) × 2 (Met) factorial arrangement with six replicates of eight chicks (half males and half females) per replicate cage (110 cm × 60 cm × 50 cm). Control starter (1–21 d) and finisher (22–42 d) diets were formulated to contain 0·50 and 0·43 % Met, respectively, according to the NRC (1994) requirements for broilers (Table 1). A high-Met treatment was formulated by adding 0·1 % dl-Met (98 %; Adisseo, Inc.) on top of the control diets (0·60 and 0·53 % Met during the starter and finisher phases, respectively). Chicks were allowed free access to mash feed and water in three-layered battery cage units in a temperature-controlled room. Continuous light was maintained, and the temperature of the experimental room was set at 32–34°C for the first 3 d and then reduced by 2–3°C per week to a final temperature of 20°C. At 42 d of age, chicks were weighed and feed consumption was recorded by replicate to calculate body weight, average daily gain, average daily feed intake and feed conversion ratio (feed intake:weight gain). Mortality was also recorded. Chicks that died during the experiment were weighed, and data were included only in the calculation of feed conversion ratio.
Table 1 Composition and nutrient content of basal diets (as-fed basis)
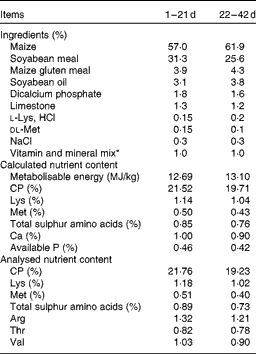
CP, crude protein.
* The premix provided per mg/kg diet: retinyl acetate, 3·44; cholecalciferol, 0·075; all-rac-α-tocopherol acetate, 30; menadione, 1·3; thiamin, 2·2; riboflavin, 8; nicotinamide, 40; choline chloride, 600; calcium pantothenate, 10; pyridoxine.HCl, 4; biotin, 0·04; folic acid, 1; cobalamin, 0·013; Fe (as FeSO4.H2O), 80; Cu (as CuSO4.5H2O), 8; Mn (as MnSO4.H2O), 110; Zn (as ZnO), 65; I (as KIO3), 1·1; Se (as Na2SeO3), 0·3.
Sample collection
At 42 d of age, one chick from each replicate was randomly selected and weighed after feed deprivation for 12 h. Chicks were killed by cervical dislocation. The whole breast (including pectoralis major and minor) muscle was weighed, and then samples were collected from the pectoralis major muscle and stored in liquid N2 until analysis.
Measurement of insulin-like growth factor-I levels in breast muscle
After thawing at room temperature, the breast muscle samples were homogenised (1:19, w/v) with an ice-cold physiological saline solution and then centrifuged at 5000 g for 10 min at 4°C. Aliquots of the supernatant were collected for subsequent assay. All determinations were carried out in duplicate. Total protein content was determined as described previously( Reference Bradford 19 ). The concentration of IGF-I was measured using a commercial chicken-specific ELISA kit (Nanjing Jiancheng Bioengineering Institute), and it is expressed as ng/mg protein.
mRNA quantification
Total RNA was isolated from breast muscle as described previously( Reference Wen, Wang and Zhou 20 ), using RNAiso reagent (TaKaRa Biotechnology). Its purity and concentration were measured using a NanoDrop ND-1000 UV spectrophotometer (NanoDrop Technologies). Later, RNA samples were diluted in diethyl pyrocarbonate-treated water to an appropriate concentration.
Reverse transcription of total RNA was carried out using a PrimeScript RT reagent Kit (TaKaRa). The geometric means of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin were used to normalise the genes of interest as recommended( Reference Vandesompele, De Preter and Pattyn 21 ). The primers for IGF-I, β-actin and GAPDH were synthesised according to the method of Li et al. ( Reference Li, Yuan and Yang 22 ), Kogut et al. ( Reference Kogut, Iqbal and He 23 ) and Wang et al. ( Reference Wang, Jiang and Tan 24 ), respectively, and those for TOR, 4EBP1, S6K1, atrogin-1, MuRF1, FOXO1 and FOXO4 were specifically designed according to the sequences in GenBank (Table 2). Quantification of mRNA was performed on an ABI 7300 Real-Time PCR System (Applied Biosystems) using SYBR Premix Ex Taq II (TaKaRa). Optimised cycling conditions for all the genes were 95°C for 30 s followed by forty cycles of 95°C for 5 s and 60°C for 31 s and a final dissociation stage of 95°C for 15 s, 60°C for 1 min, 95°C for 15 s and 60°C for 15 s. All measurements were carried out in triplicate, and average values were obtained. Relative mRNA levels (arbitrary units) were calculated on the basis of PCR efficiency and threshold cycle (C t) values as described previously( Reference Pfaffl 25 ). The mRNA level of each target gene in heavy chicks fed the control diets was assigned a value of 1.
Table 2 Sequences used for real-time PCR primers

IGF-I, insulin-like growth factor-I; TOR, target of rapamycin; 4EBP1, eIF4E-binding protein 1; S6K1, ribosomal protein S6 kinase 1; MuRF1, muscle ring finger-1; FOXO, forkhead box O; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
Two-way ANOVA was employed to determine the main effects of HW and Met and their interaction using the general linear model procedure of SPSS software (version 16.0; SPSS, Inc.). Differences among the treatments were examined by one-way ANOVA using Duncan's multiple range test, which were considered significant at P< 0·05, and P values between 0·05 and 0·1 were considered as a trend. Data are presented as means with their pooled standard errors.
Results
Growth performance
Mortality was low (3 %) and not related to treatment (data not shown). Heavy chicks had higher (P< 0·05) 42 d body weight and average daily gain than light chicks when both were fed the control diets, and feed conversion ratio showed a decreasing trend (P= 0·094) (Table 3). High-Met diets improved (P< 0·05) 42 d body weight, average daily gain and feed conversion ratio in light chicks but not in heavy chicks (HW × Met interaction; P< 0·05). The performance of light chicks fed high-Met diets was similar to that of heavy chicks fed either diet. There was no difference in average daily feed intake among the groups.
Table 3 Effect of methionine (Met) levels on the performance of broilers with different hatching weights (HW) from 1 to 42 d of age (Mean values with their standard errors, n 6, 8 chicks per replicate)
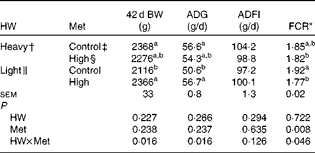
BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio.
a,bMean values within a column with unlike superscript letters were significantly different (P< 0·05).
* FCR = feed intake:weight gain.
† Mean hatching weight of 48·3 (sem 0·1) g.
‡ 0·50 and 0·43 % Met during the starter (1–21 d) and finisher (22–42 d) phases, respectively.
§ 0·60 and 0·53 % Met during the starter (1–21 d) and finisher (22–42 d) phases, respectively.
∥ Mean hatching weight of 41·7 (sem 0·1) g.
Breast muscle weight and insulin-like growth factor-I concentration
The absolute weight of breast muscle and concentration of IGF-I were lower (P< 0·05) in light chicks than in heavy chicks when both were fed the control diets, and the same trend was observed for relative weight (P= 0·067) (Table 4). High-Met diets increased (P< 0·05) the absolute and relative weights of breast muscle as well as concentration of IGF-I in light chicks but not in heavy chicks (HW × Met interaction; P< 0·05).
Table 4 Effect of methionine (Met) levels on breast (including pectoralis major and minor) muscle weight and insulin-like growth factor-I (IGF-I) concentration of broilers with different hatching weights (HW) at 42 d of age (Mean values with their standard errors, n 6, 8 chicks per replicate)

BW, body weight.
a,bMean values within a column with unlike superscript letters were significantly different (P< 0·05).
* Mean hatching weight of 48·3 (sem 0·1) g.
† 0·50 and 0·43 % Met during the starter (1–21 d) and finisher (22–42 d) phases, respectively.
‡ 0·60 and 0·53 % Met during the starter (1–21 d) and finisher (22–42 d) phases, respectively.
§ Mean hatching weight of 41·7 (sem 0·1) g.
mRNA expression
The expression of IGF-I mRNA was lower (P< 0·05) in light chicks than in heavy chicks when both were fed the control diets, while that of other genes tested did not differ (P>0·10; Table 5). High-Met diets up-regulated (P< 0·05) the mRNA levels of IGF-I and TOR and down-regulated those of 4EBP1, atrogin-1 and FOXO4 in light chicks (P< 0·05), but no difference was observed in heavy chicks. Treatments did not affect the expression of MuRF1, S6K1 or FOXO1 mRNA.
Table 5 Effect of methionine (Met) levels on the relative mRNA levels* in the breast muscle of broilers with different hatching weights (HW) at 42 d of age (Mean values with their standard errors, n 6, 8 chicks per replicate)
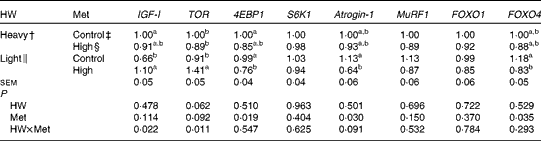
IGF-I, insulin-like growth factor-I; TOR, target of rapamycin; 4EBP1, eIF4E-binding protein 1; S6K1, ribosomal protein S6 kinase 1; MuRF1, muscle ring finger-1; FOXO, forkhead box O.
a,bMean values within a column with unlike superscript letters were significantly different (P< 0·05).
* The mRNA level of each target gene in heavy chicks fed the control diets was assigned a value of 1 (arbitrary units).
† Mean hatching weight of 48·3 (sem 0·1) g.
‡ 0·50 and 0·43 % Met during the starter (1–21 d) and finisher (22–42 d) phases, respectively
§ 0·60 and 0·53 % Met during the starter (1–21 d) and finisher (22–42 d) phases, respectively.
∥ Mean hatching weight of 41·7 (sem 0·1) g.
Discussion
The present study confirmed that heavy chicks had better performance than light ones when both were fed the control diets, as reported previously( Reference Mendes, Paixão and Restelatto 7 , Reference Sklan, Heifetz and Halevy 9 ). High-Met diets improved the performance of light chicks, which was similar to that of heavy chicks, indicating that Met levels used in the control diets in the present study were adequate for heavy chicks but inadequate for light chicks. Similar results were obtained by Leandro et al. ( Reference Leandro, Cunha and Stringhini 5 ), who reported that performance from 1 to 40 d of age did not differ between broilers with HW of 40·4 (sem 0·5) and those with HW of 49·3 (sem 1·1) g, when high Met amounts were included in the diets (0·61 % for 1–7 d, 0·57 % for 8–21 d and 0·54 % for 22–40 d). This implied that the Met requirement of broilers might depend, at least in part, on their HW and that those with lower HW might need more Met supply to achieve their growth potential.
In the present study, light chicks fed the control diets had lower breast muscle weight at 42 d of age. This finding is in agreement with the results of Sklan et al. ( Reference Sklan, Heifetz and Halevy 9 ). The concentration of IGF-I in the breast muscle of light chicks followed a similar pattern, which suggests that differences in breast muscle growth might be due to variations in IGF-I synthesis( Reference Sklan, Heifetz and Halevy 9 , Reference Guernec, Berri and Chevalier 26 ). Breast muscle weight and IGF-I concentration of light chicks were promoted by high-Met diets, suggesting that Met may improve breast muscle growth by enhancing IGF-I synthesis. The improvement of breast meat yield by high-Met diets has been reported previously( Reference Hickling, Guenter and Jackson 13 , Reference Ahmed and Abbas 27 ). However, there is little literature on the response of breast muscle IGF-I content to Met levels in broiler diets. The response of plasma IGF-I levels to dietary Met has been reported previously( Reference Carew, McMurtry and Alster 28 ), but as Nagao et al. ( Reference Nagao, Oki and Tsukada 29 ) reported, the regulatory effect of dietary Met was independent of the change in plasma IGF-I concentration. The lack of response in heavy chicks could be attributed to the fact that these chicks had greater muscle mass with more satellite cells that underwent higher proliferation and earlier differentiation after hatching( Reference Sklan, Heifetz and Halevy 9 ) and thus were less sensitive to high-Met diets.
As a first step in the elucidation of the mechanism by which breast muscle growth is regulated by Met, the mRNA levels of genes related to the IGF-I signalling pathway were measured in the present study. The levels of IGF-I mRNA in breast muscle were lower in light chicks than in heavy ones when both were fed the control diets, supporting the hypothesis that IGF-I mRNA may participate in the setting of muscle growth rate during development( Reference Guernec, Berri and Chevalier 26 ). High-Met diets increased IGF-I mRNA levels in light chicks, which was parallel to the changes in its concentration. Nutrient supply has been reported to enhance the expression of IGF-I mRNA in chicken skeletal muscle( Reference Li, Yuan and Yang 22 , Reference Guernec, Chevalier and Duclos 30 ), but no data are available on its response to dietary Met. Increased TOR and decreased 4EBP1 mRNA levels without any change in the expression of S6K1 mRNA in light chicks fed high-Met diets imply that the TOR/4EBP1 pathway may be regulated by Met at the transcriptional level. Further work is required to determine whether the phosphorylation of these proteins is involved in this process. The present findings are not consistent with those of Wang et al. ( Reference Wang, Jiang and Tan 24 ), who reported that decreasing dietary nutrient density increased the levels of TOR, 4EBP1 and S6K1 mRNA in the gastrocnemius muscle but not in the pectoralis major muscle of slow-growing chickens. This discrepancy may be related to broiler strains and muscle types. The reduction in the expression of atrogin-1 mRNA without any change in that of MuRF1 in the breast muscle of light chicks fed high-Met diets indicates that Met may improve the muscle growth of light chicks by preventing the down-regulation of protein synthesis but not proteolysis( Reference Foletta, White and Larsen 31 ). Met supply has been reported to modulate the expression of atrogin-1 in quail muscle fibroblasts( Reference Tesseraud, Métayer-Coustard and Boussaid 32 ). In other studies, the expression of atrogin-1 mRNA has been reported to be increased in chickens fed low-lysine diets( Reference Tesseraud, Bouvarel and Collin 18 ) or subjected to fasting( Reference Nakashima, Yakabe and Yamazaki 33 ), showing that the expression of atrogin-1 is affected by nutritional status. A change in FOXO4 mRNA expression that was the same as that in atrogin-1 mRNA expression suggests that Met may regulate the expression of atrogin-1 by inhibiting FOXO4, which is probably induced by the enhanced expression of IGF-I and associated signalling pathway( Reference Stitt, Drujan and Clarke 15 ). Parallel changes in the expression of atrogin-1 and FOXO4 mRNA have been observed in growing rats fed diets with different amino acid profiles( Reference Luo, Chen and Yu 34 ). Previous research has shown the regulatory effect of FOXO4 on the expression of atrogin-1 ( Reference Moylan, Smith and Chambers 35 ). No difference in the expression of FOXO1 mRNA suggests that the response to dietary Met is isoform specific, with FOXO4 being more sensitive. This may be explained by the differential expression level of these isoforms between different organs; for example, FOXO4 is highly expressed in muscle, whereas FOXO1 is highly expressed in adipose tissue( Reference Burgering 17 ).
In conclusion, Met levels used in the control diets in the present study were adequate for heavy chicks but inadequate for light chicks, resulting in poorer performance and breast muscle growth, which were improved by increasing dietary Met supply probably through alterations in IGF-I synthesis and gene expression of the TOR/4EBP1 and FOXO4/atrogin-1 pathway.
Acknowledgements
The authors thank their laboratory colleagues for their assistance.
The authors acknowledge Anhui Hewei Agricultural Development Company Limited (Guangde, Xuancheng, Anhui, China) for providing the chicks without charge. The company had no role in the design, analysis or writing of the article.
The contributions of the authors are as follows: P. W. and Y. C. carried out the experiments together with C. W., who also performed the data analysis and wrote the manuscript; Y. Z. and T. W. designed and supervised the study and revised the manuscript.
The authors had no conflicts of interest.







