The observation that high egg consumption is strongly associated with incident gestational diabetes suggests that egg consumption may disrupt glucose metabolism( Reference Qiu, Frederick and Zhang 1 ). Eggs are a major source of cholesterol, and in animal models cholesterol intake disrupts glucose metabolism and increases circulating insulin( Reference Adamopoulos, Papamichael and Zampelas 2 ). In humans, dietary cholesterol appears to affect type 2 diabetes risk( Reference Salmeron, Hu and Manson 3 ), and elevated cholesterol intake prior to pregnancy is associated with an increased risk for gestational diabetes( Reference Bowers, Tobias and Yeung 4 ). Few prospective studies have evaluated the relation between egg consumption and incidence of type 2 diabetes. Among middle-aged US adults, men and women who consumed at least one egg a day had a 58 and a 77 % higher rate of diabetes compared with those who did not consume eggs( Reference Djousse, Gaziano and Buring 5 ). This relation was not observed in a cohort of Adventists( Reference Vang, Singh and Lee 6 ), in older US adults( Reference Djousse, Kamineni and Nelson 7 ), or in two European( Reference Zazpe, Beunza and Bes-Rastrollo 8 , Reference Virtanen, Mursu and Tuomainen 9 ) and a Japanese( Reference Kurotani, Nanri and Goto 10 ) population. Such inconsistency could be explained by differences in the distribution of egg consumption, in the variables used for adjustment for confounding, in the method of preparation or in the foods consumed in conjunction with eggs. Therefore, we investigated the relation between egg and cholesterol consumption and incident type 2 diabetes in a large prospective cohort of French women.
Methods
Study population
The E3N study (Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education Nationale) is a French prospective study of 98 995 women born between 1925 and 1950 and affiliated to a health insurance plan that covers mostly teachers and their spouses that began in 1990. E3N is the French component of the European Prospective Investigation into Cancer and Nutrition. Cohort members have returned mailed questionnaires on reproductive and lifestyle information and on newly diagnosed diseases in 1992, 1993, 1994, 1997, 2000, 2002, 2005 and 2008. Loss to follow-up has been low (3 %), and average follow-up in each questionnaire cycle has been 83 %. All women signed an informed consent form in compliance with the French National Commission for Computerized Data and Individual Freedom. In 1993, 74 154 women responded to a follow-up questionnaire and a validated self-administered diet history questionnaire( Reference van Liere, Lucas and Clavel 11 ). We excluded those with an unrealistic energy consumption (n 1366), no follow-up (n 720), prevalent diabetes (n 1576), diabetes with no date of diagnosis (n 81), prevalent cancer or CVD (n 4950) and women who reported following a diet for diabetes (n 97). The final study population comprised 65 364 women.
Dietary and covariate assessment
Between 1993 and 1995, dietary data were collected using a previously validated 208-item self-administered diet history questionnaire, with eleven categories of frequency: never or less than once a month; one, two or three times a month; and one to seven times a week. Total egg consumption was based on the number and frequency of consumption of hardboiled eggs and of eggs consumed in omelettes or mixed dishes. Nutrient intakes, including that of dietary cholesterol, were calculated using a food composition table derived from a French national database. The validity and reproducibility of the dietary questionnaire was previously assessed in a sample of 119 women who had completed two diet history questionnaires and twelve monthly 24-h dietary recalls over a 1-year period( Reference van Liere, Lucas and Clavel 11 ). The correlation coefficient between the dietary questionnaire and the 24-h recalls was 0·49 for eggs and 0·40 for dietary cholesterol. Education was assessed in 1990 at cohort recruitment with the following categories: no studies, primary-school certificate, secondary-school certificate, high-school certificate (baccalaureate) to 2-year post-high-school education, 3 to 4 years of post-high-school education and 5 or more years of post-high-school education. We defined university-educated individuals as those who responded to the last two categories. For other covariates, we used information from a questionnaire sent to participants along with the dietary assessment. Treated hypertension and hypercholesterolaemia, and smoking were based on self-reports. Menopausal status was determined using information on last menstruation, the presence of hot flushes, and a history of hysterectomy, ovariectomy and menopausal hormonal treatments. Self-reported height and weight were used to calculate BMI, defined as weight (kg) divided by height squared (m2). In this cohort, self-reported anthropometry is reliable (in a sub-study the correlation coefficient between self-reported and measured height was 0·89 m2 and for weight was 0·94 kg)( Reference Tehard, van Liere and Com Nougue 12 ). We assessed usual physical activity with a questionnaire that included items on weekly hours spent walking, cycling, performing light and heavy household chores and recreational activities (e.g. swimming, tennis) during the summer and the winter. This questionnaire performed well in a previous study( Reference Tehard, Friedenreich and Oppert 13 ).
Ascertainment of type 2 diabetes
As previously described( Reference Lajous, Bijon and Fagherazzi 14 ), for case identification, we used self-reports, supplementary questionnaires and drug reimbursement information. Between 1993 and 2008, 2597 self-reported incident cases were confirmed either by a supplementary questionnaire or by drug reimbursement claims’ database, and an additional 843 incident cases only through this database, but validated by a supplementary questionnaire. The present analysis is based on incident cases of diabetes after applying the exclusion criteria detailed above (no date of diagnosis, unavailable dietary information or unrealistic energy consumption, no follow-up, prevalent cancer or CVD and a diet for diabetes at baseline).
Statistical analysis
Nutrients were energy-adjusted using the residual method( Reference Willett 15 ). We categorised egg consumption as 0, <1, 1–1·9, 2–4·9 and ≥5 eggs/week, and cholesterol in quintiles, and used the lowest category as the referent. To test for trend, we used the median value for each category as a continuous variable. We evaluated cholesterol consumption using a substitution model for carbohydrates that included intakes of energy, saturated fat, unsaturated fat and protein (excluding carbohydrates) in the model. We calculated person-time from the date of completion of the dietary questionnaire to the date of diagnosis of diabetes, last follow-up available or June 2008. Hazard ratios (HR) and 95 % CI were estimated from Cox regression models with age as the time scale (SAS 9.1; SAS Institute Inc.). Models were adjusted for education, menopause, menopausal hormone therapy, treated hypertension and hypercholesterolaemia, physical activity, smoking, total energy, alcohol, processed red meat, coffee, fruit, vegetables, sugar and artificially sweetened beverages and BMI. We hypothesised that BMI may modify the association between dietary variables and diabetes; therefore, we tested for statistical interaction by including a cross-product term of median egg and cholesterol consumption by BMI (<25, ≥25 kg/m2) in the Cox model and by performing a log-likelihood ratio test.
Results
Mean age was 52·7 (sd 6·6) years; the prevalence of overweight was 15·5 % and that of obesity was 3·2 %. Mean total egg consumption was 3·5 (sd 2·8) eggs/week, and the mean weekly hardboiled and omelette/mixed-dish egg intakes were 1·0 (sd 1·5) and 2·5 (sd 2·1), respectively. Mean cholesterol intake was 378 (sd 104) mg/d, and the main food contributors of dietary cholesterol were omelette/mixed-dish egg (17 %), hardboiled egg (7 %), butter (4 %), liver (4 %), pizza and quiche (3 %), poultry (3 %), creams, dairy dessert and ice creams (3 %), beef (3 %) and cheese (3 %). The correlation coefficient for dietary cholesterol with saturated fat was 0·24 (P value<0·0001) and −0·26 (P value<0·0001) with dietary fibre. Egg consumption and cholesterol intake were directly related to intake of alcohol, processed red meat and coffee and to BMI and hypertension. We observed an inverse relation with treated hypercholesterolaemia and education (Table 1).
Table 1 Age-standardised risk factors for diabetes risk by total egg consumption and dietary cholesterol quintiles in a cohort of French women in 1993 (Mean values and standard deviations; percentages)

MHT, menopausal hormone therapy.
We identified 1803 incident diabetes cases during the study period. After multivariable adjustment, the HR comparing women who consumed ≥5 eggs/week with non-consumers was 1·00 (95 % CI 0·78, 1·29), with a P trend=0·11 (Table 2). The corresponding values for hardboiled egg consumption were HR 1·16 (95 % CI 0·89, 1·50; P trend=0·12). For omelette/mixed-dish eggs these values were HR 0·91 (95 % CI 0·73, 1·14; P trend=0·67). For dietary cholesterol, women in the highest quintile of intake (median, 507 mg) had a 40 % higher rate of diabetes as compared with those in the lowest quintile (median, 241 mg) (HR 1·40; 95 %CI 1·19, 1·63; P trend<0·0001) (Table 3). There was an attenuation when results were adjusted for BMI; however, the relations remained statistically significant. In the substitution model, each additional 100 mg dietary cholesterol per 4184 kJ (or 1000 kcal) was associated with a 14 % increase in the risk for diabetes (HR 1·14; 95 % CI 1·02, 1·26). These associations did not differ between normal weight (BMI<25 kg/m2) and overweight individuals (BMI≥25 kg/m2). The P values for interaction were 0·36 for egg consumption and 0·11 for cholesterol intake. In sensitivity analyses, we stratified models on hypertension and hypercholesterolaemia. The association remained in hypertensive and non-hypertensive women. We observed a slightly stronger association among hypertensive women (comparing extreme quintiles: HRhypertensive=1·58; 95 % CI 1·11, 2·26, v. HRnon-hypertensive=1·38; 95 % CI 1·15, 1·65); however, there was no evidence of heterogeneity (P interaction=0·91). For hypercholesterolaemia, there was no statistical evidence of a difference in the association of dietary cholesterol with diabetes. HR for the comparison of extreme categories of dietary cholesterol were 1·19 (95 % CI 0·76, 1·86; P trend=0·24) and 1·44 (95 % CI 1·21, 1·71; P trend<0·0001) in women with and without hypercholesterolaemia, respectively (P interaction=0·21).
Table 2 Age-adjusted and multivariable-adjusted hazard ratios (HR) of type 2 diabetes according to the number of eggs consumed per week in the Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education Nationale cohort study (1993–2008) (Hazard ratios and 95 % confidence intervals)
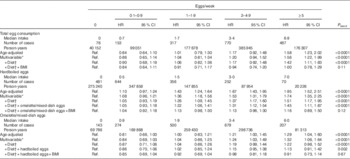
Ref., referent values.
* Adjusted for education, smoking (never, secondhand smoke, past, current), physical activity (h/week, quartiles), hormone replacement therapy (premenopausal, current, past, never), hypertension, hypercholesterolaemia and energy (quintiles).
† Additional adjustment alcohol (quintiles), processed red meat (quintiles), coffee (quintiles), fruits (quintiles), vegetables (quintiles), sugar-sweetened drinks (no consumers+quartiles) and artificially sweetened drinks (no consumers+quartiles).
Table 3 Age-adjusted and multivariable-adjusted hazard ratios (HR) of type 2 diabetes according to the quintiles of dietary cholesterol in the Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education Nationale cohort study (1993–2008) (Hazard ratios and 95 % confidence intervals)
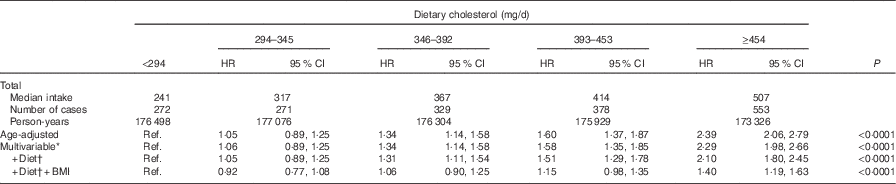
Ref., referent values.
* Adjusted for education, smoking (never, secondhand smoke, past, current), physical activity (h/week, quartiles), hormone replacement therapy (premenopausal, current, past, never), hypertension, hypercholesterolaemia and energy (quintiles).
† Additional adjustment alcohol (quintiles), processed red meat (quintiles), coffee (quintiles), fruits (quintiles), vegetables (quintiles), sugar-sweetened drinks (no consumers+quartiles) and artificially sweetened drinks (no consumers+quartiles).
Discussion
After a median 13·8-year follow-up, dietary cholesterol was found to be associated with a risk for type 2 diabetes in a prospective study on French women. We found no overall association with egg consumption.
Animal studies suggest that diets rich in fat( Reference Wu, Vikramadithyan and Yu 16 ) and egg consumption( Reference Adamopoulos, Papamichael and Zampelas 2 ) may disrupt glucose metabolism and increase circulating insulin. In addition, data from different sources suggest that the cholesterol metabolism may contribute to diabetes risk( Reference Brunham, Kruit and Verchere 17 ). Our results confirm a previous observation of a 36 % higher diabetes risk in a large cohort of US women on comparing women with the highest and the lowest dietary cholesterol intakes( Reference Salmeron, Hu and Manson 3 ). In a study that included a prospective cohort and a case–control study of maternal diet, there was a more than 2-fold higher risk for gestational diabetes on comparing extreme categories of dietary cholesterol( Reference Qiu, Frederick and Zhang 1 ). However, in a cohort study in Japan, dietary cholesterol was not associated with diabetes incidence after a 5-year follow-up( Reference Kurotani, Nanri and Goto 10 ). In contrast to Western populations, in this study, red meat was not the most common food source for dietary cholesterol. Therefore, it is possible that the discrepancy may be due to differences in the distribution of cholesterol food sources. In E3N, we previously observed a relation between processed red meat and diabetes( Reference Lajous, Tondeur and Fagherazzi 18 ); thus, we adjusted for processed red meat in our models. In addition, it is possible that we may have been able to detect an association because the distribution of cholesterol was wider in our study. The mean cholesterol intake in women in Japan for the highest category was 390 mg/d, whereas in the present study the median intake for the highest quintile of intake was 507 mg/d. A recent analysis in the smaller Kuopio prospective cohort did not show an association between cholesterol intake, assessed using 4-d food records and incident diabetes after a 19-year follow-up( Reference Virtanen, Mursu and Tuomainen 9 ). Individuals may have changed their diet over the follow-up period, potentially attenuating the association. In a sensitivity analysis restricted to the first 10 years of follow-up, cholesterol intake appeared to confer risk of diabetes; however, results were not statistically significant. For egg consumption, our results differ from observations reported in a meta-analysis based on four prospective US studies( Reference Shin, Xun and Nakamura 19 ) that found an association between egg consumption and type 2 diabetes and also from a study on maternal diet and gestational diabetes( Reference Qiu, Frederick and Zhang 1 ). More recently, this association was evaluated in a small Spanish cohort study with relatively few incident cases of diabetes( Reference Zazpe, Beunza and Bes-Rastrollo 8 ) and in the Kuopio study in Finland( Reference Virtanen, Mursu and Tuomainen 9 ); no association was observed in either study. Eggs are often consumed with other foods, and there are several methods of preparation that include the addition of other nutrients. Thus, other foods or added nutrients may be responsible for the observed relation. In our study, we did not observe a relation between egg consumption and diabetes risk after adjustment for BMI, a variable that is known to be strongly associated with diabetes and is also associated with diet.
The prospective design, limited loss to follow-up, use of a validated dietary questionnaire and the possibility of evaluating different egg preparations separately are important strengths of the present analysis. There are some limitations to consider. First and foremost, there is some evidence for potential biological mechanisms supporting altered cholesterol metabolism and diabetes risk. However, it is less clear how dietary cholesterol could affect diabetes occurrence. Diet was assessed only at baseline, and participants may have changed their diet during follow-up. Thus, a non-differential measurement error is possible and could potentially explain null results. Other dietary analyses in this cohort have shown significant associations with diabetes and could be indicative that this error may not be substantial. Non-differential misclassification of diabetes is also a possibility. However, assuming very few false positives, this error probably had no measurable effect on our estimates. Confounding by unmeasured factors such as dietary factors prior to baseline, or by variables that are difficult to measure, such as unhealthy behaviour, can never be ruled out. We adjusted for the most important risk factors for diabetes and saw a large attenuation of HR. We further explored this possibility by stratifying analyses on hypercholesterolaemia and hypertension, two diagnoses that could be related to unhealthy behaviours and could also alter dietary habits. However, the association between dietary cholesterol and diabetes remained among individuals without these diagnoses.
Our results suggest that dietary cholesterol is associated with type 2 diabetes, but habitual egg consumption is not. These results should be interpreted with caution as the underlying mechanism for the relation of dietary cholesterol and diabetes is unclear, and there is potential for residual confounding. Future studies should address potential mechanisms by which glucose metabolism may be disrupted by cholesterol intake.
Acknowledgements
The authors are indebted to the participants in the Etude Epidémiologique auprès des femmes de la Mutuelle Générale de l’Education Nationale (E3N) for their continuing dedication and support. The authors thank Thierno Mamadou Bah for additional statistical support.
The E3N cohort study was carried out with the financial support of the Mutuelle Générale de l’Education Nationale, European Community, French League against Cancer, Gustave Roussy Institute and French Institute of Health and Medical Research. The validation of potential diabetes cases was supported by the European Union (Integrated Project LSHM-CT-2006-037197 in the Framework Programme 6 of the European Community) InterAct project. The present analysis was conducted with the support of the International Associated Laboratory in Nutrition, Obesity, Hormones and Cardiometabolic Disease and Depression, an ongoing collaboration between the National Institute of Health and Medical Research (Inserm, France) and the National Institute of Public Health (INSP, Mexico). M. L. is a Bernard Lown Visiting Scholar in Cardiovascular Health at the Harvard School of Public Health.
M. L. formulated the research question. M. L., A. B., M.-C. B.-R. and F. C.-C. designed the study; M. L., A. B., M.-C. B.-R. and G. F. conducted research; A. B. analysed the data; M. L., B. B. and M.-C. B.-R. and F. C.-C. wrote the paper; M. L. had primary responsibility for final content. All authors read and approved the final manuscript.
There are no conflicts of interest.






