Introduction
Neobenedenia species (Monogenea: Caspsalidae) are marine parasites of finfish that cause major epidemics in marine aquaculture (Whittington, Reference Whittington, Woo and Buchmann2012). Species in this genus are reported to affect commercial cultivation of orange-spotted grouper Epinephelus coioides, John's snapper Lutjanus johnii, mangrove red snapper Lutjanus argentimaculatus and pinjalo Pinjalo pinjalo in South-East Asia (Seng, Reference Seng1997); greater amberjack Seriola dumerili, almaco jack Seriola rivoliana, Japanese amberjack Seriola quinqueradiata, bastard halibut Paralichthys olivaceus and the spotted halibut Verasper variegatus in Japan (Ogawa et al., Reference Ogawa, Bondad-Reantaso, Fukudome and Wakabayashi1995; Hirayama et al., Reference Hirayama, Kawano and Hirazawa2009; Ohno et al., Reference Ohno, Kawano and Hirazawa2009; Hirazawa et al., Reference Hirazawa, Tsubone and Takano2016; Sicuro & Luzzana Reference Sicuro and Luzzana2016); barramundi Lates calcarifer in Indonesian and Australian aquaculture (Seng, Reference Seng1997; Hutson et al., Reference Hutson, Mata, Paul and de Nys2012); and yellowtail amberjack Seriola lalandi in Mexico (Avilés-Quevedo & Castello-Orvay, Reference Avilés-Quevedo and Castello-Orvay2004).
Neobenedenia spp. are recorded from over 100 species in 30 families from five different orders from wild, aquarium and farmed teleosts worldwide (Wittington & Horton, Reference Whittington and Horton1996), thus prevention of parasitic infections with Neobenedenia spp. is difficult. The parasite exhibits a direct life cycle; adult Neobenedenia sp. attach to the host skin and lay filamentous eggs into the water, which hatch into ciliated larvae (oncomiracidia) that can re-infect rapidly (Whittington, Reference Whittington, Woo and Buchmann2012), initially attaching opportunistically to fish and then migrating to specific microhabitats (Trujillo-González et al., Reference Trujillo-González, Constantinoiu, Rowe and Hutson2015). In addition, eggs can entangle with each other and accumulate on the shallow areas of sea-cage nets (Shirakashi & Hirano, Reference Shirakashi and Hirano2015). The typical management methods used for monogeneans are baths with freshwater, hydrogen peroxide or formalin (Thoney & Hargis, Reference Thoney and Hargis1991). All of these treatments kill adult parasites, but leave the fish vulnerable to re-infection (Diggles et al., Reference Diggles, Roubal and Lester1993; Yoshinaga et al., Reference Yoshinaga, Segawa, Kamaishi and Sorimachi2000). Oral administration of the anthelmintic praziquantel has been tested to treat monogenean parasites infecting fish and is registered for use in Japan (Forwood et al., Reference Forwood, Bubner, Landos, D'Antignana and Deveney2016); however, the drug affects the palatability of the feed and consequently the efficacy of the treatment (Partridge et al., Reference Partridge, Burge and Lymbery2017).
Herbal medicines are being examined in aquaculture research as an alternative method for disease management. More than 250 plant species from 75 families and 32 orders, which can be applied orally, through immersion or intraperitoneal injection (Bulfon et al., Reference Bulfon, Volpatti and Galeotti2015), have been studied to evaluate their use as growth promoters, for prophylactic and therapeutic control methods, and as immune system modulators (Awad & Awaad, Reference Awad and Awaad2017; Reverter et al., Reference Reverter, Tapissier-Bontemps, Sasal, Saulnier, Austin and Newaj-Fyzul2017). The lethal effect of most plant extracts on disease agents is attributed to secondary metabolites, e.g. saponins, alkaloids, tannins, phenolics, polyphenols, lignins, glycosides and polypeptide compounds (Van Hai, Reference Van Hai2015). Secondary metabolites have been selected by plants during evolution to fulfil a chemical defence mechanism or to act as signalling compounds in plant–animal, plant–microbe and plant–plant interactions. Structures of secondary metabolites have been identified to interfere in three main areas: in proteins, where they can act as agonists (stimulating receptors) or antagonists (receptor blockers); in DNA and RNA, including related enzymes and regulatory proteins; and in biomembranes (Wink, Reference Wink2008). Most research examining the efficacy of herbal medicines in freshwater fish species has been concerned with bacterial diseases (Citarasu, Reference Citarasu2010; Ramudu & Dash, Reference Ramudu and Dash2013; Reverter, et al., Reference Reverter, Bontemps, Lecchini, Banaigs and Sasal2014; Vaseeharan & Thaya, Reference Vaseeharan and Thaya2014) and few studies have examined the antiparasitic effect of plants on metazoan parasite infections. Garlic-based treatments have been shown to prevent Gyrodactylus spp. infection in Oreochromis niloticus fry (Abd El-Galil & Aboelhadid, Reference Abd El-Galil and Aboelhadid2012) and to impede significantly hatching success, oncomiracidia longevity and infection success of Neobenedenia sp. infecting L. calcarifer (Militz et al., Reference Militz, Southgate, Carton and Hutson2013a, Reference Militz, Southgate, Carton and Hutsonb). Exposure to garlic and ginger extract reduced infection with Gyrodactylus turnbulli in guppy Poecilia reticulata (Fridman et al., Reference Fridman, Sinai and Zilberg2014; Levy et al., Reference Levy, Zilberg, Paladini and Fridman2015).
Therefore, this study investigated the efficacy of six plant extracts – garlic (Allium sativum), ginger (Zingiber officinale), basil (Ocimum basilicum), bitter chaparro (Castela tortuosa), onion (Allium cepa) and papaya (Carica papaya) – against the three parasite life stages (adults, eggs and oncomiracidia) of Neobenedenia sp. in vitro, to identify potential natural treatments for the management of monogenean ectoparasites in aquaculture.
Materials and methods
Source and identification of Neobenedenia
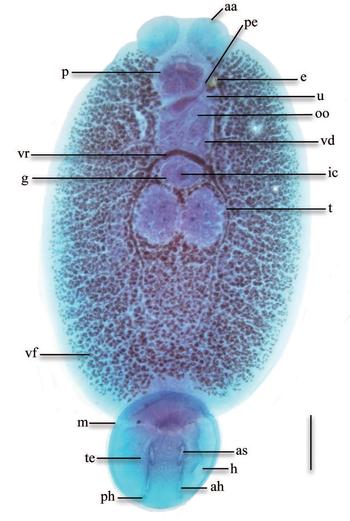
Eighty yellowtail amberjack S. lalandi Valenciennes (average weight 150 ± 50 g) were obtained from marine cages from a local company ©Baja Seas, at Bahia Magdalena BCS, Mexico. Fish were confirmed to be infected by one species of skin parasite. Parasites were fixed in formalin 10%, stained with Gomori's trichrome and mounted in synthetic resin. Adult parasites were identified as Neobenedenia (fig. 1) using morphological criteria: lack of vagina, pair of haptor extrinsic muscles in body with long tendons entering haptor, presence of marginal valve and two disc-shaped anterior attachment organs (Whittington & Horton, Reference Whittington and Horton1996). Species differentiation could not be made for the purpose of this study, due to the lack of morphologically distinguishable characters between species and a proposed change to reinstate currently synonymized taxa (Brazenor et al., in prep.). Subsequently, the species of this study will be referred as Neobenedenia sp. from S. lalandi off the Pacific coast of Mexico. Mounted specimens were accessioned in the Colección Nacional de Helmintos, Universidad Nacional Autónoma de México (CNHE 10581).
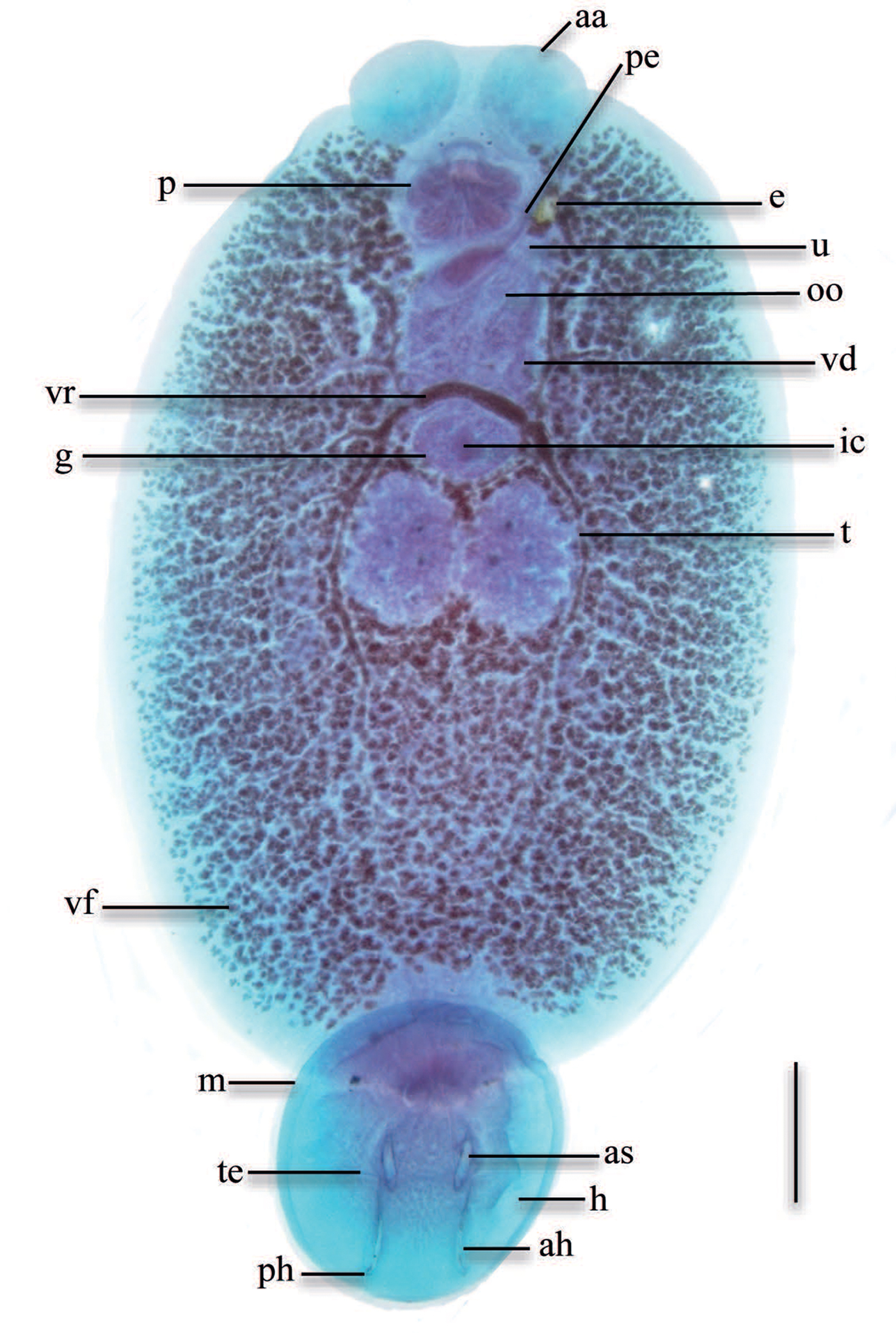
Fig. 1. Ventral view of an adult Neobenedenia sp., stained with Gomori's trichrome. Scale bar = 500 μm. aa, anterior attachment organ; ah, anterior hamulus; as, accessory sclerite; e, egg; g, germarium; h, haptor; m, marginal valve; ic, internal fertilization chamber; oo, ootype; p, pharynx; pe, penis; ph, posterior hamulus; t, testis; te, tendon; u, uterus; vd, vas deferens; vf, vitelline follicle; vr, vitelline reservoir.
To provide the source of parasites for experiments, a laboratory infection was established. Seriola lalandi was maintained in four 600-litre tanks containing five fish each, with flow-through filtered seawater (salinity 36 ± 1 g l–1, dissolved oxygen 5.5 ± 0.5 mg l–1, temperature 26 ± 1°C and natural photoperiod). Fish were fed twice a day with a commercial diet (EWOS Canada Ltd., Vancouver, British Columbia, Canada), the daily feeding rate was ~3% of body weight. Parasite eggs were obtained by using a single multifilament nylon thread (0.3 cm diameter and 30 cm length) suspended in the water, tied to the air pipe. Every 24 h the thread was examined under a stereomicroscope. The number of eggs on the thread was used as an indication of the infection level (i.e. low, <100 eggs/thread; medium, 100–499 eggs/thread; and high infection >500 eggs/thread). When the infection level was low (i.e. <100 eggs/thread), a re-infection protocol was carried out following Hirayama et al. (Reference Hirayama, Kawano and Hirazawa2009).
Preparation of treatments
Six plant extracts were selected, based on their potential anthelmintic properties: garlic A. sativum, ginger Z. officinale, basil O. basilicum, bitter chaparro C. tortuosa, onion A. cepa and papaya C. papaya. All extracts were water–ethanol (70%) solutions purchased from Extractos Sigma (Cuautitlán Izcalli, Mexico State, Mexico) (20% of dry ground matter/l of extract). Extracts were tested at three dilutions: 1:10, 1:50 and 1:100 made with filtered seawater (35 g l–1). To determine whether the ethanol (70%) component of the extracts showed toxicity toward Neobenedenia life stages, a treatment with ethanol (70%) at the dilutions of 1:10 (7% ethanol), 1:50 (1.4% ethanol) and 1:100 (0.07% ethanol) was included. Dilutions were made with seawater (35 g l–1). All treatments were compared to seawater 35 g l–1 (control), to obtain reference values under the same experimental conditions.
Adult parasite survival, egg production and time to detachment
To obtain adult parasites (on average 10 days old at 26°C), fish from laboratory infection were anaesthetized with a solution of eugenol (3 ml (100 l)–1, a natural anaesthetic recommended for parasitological studies) (Boijink et al., Reference Boijink, Maciel, Tavares-Dias, Iwashita, Morais, Hide, Souza, Couto, Meneses, Cunha and Fujimoto2016). Live parasites were carefully removed with a needle and scalpel from the surface of the host fish and transferred to a six-well flat-bottom culture vessel (15.5 ml; Becton Dickinson Labware, Franklin Lakes, New Jersey, USA). Parasites attached immediately to the bottom of the culture vessel using their haptor/anterior attachment organs (Whittington et al., Reference Whittington, Cribb, Hamwood and Halliday2000). The effect of 24 h continuous immersion in each plant extract and ethanol (70%) at three dilutions (1:10, 1:50 and 1:100) was assessed on the survival, egg production and time for parasites to detach from the plate. Death was defined as the parasite showing no signs of motion and failing to respond to tactile and light stimuli (Fridman et al., Reference Fridman, Sinai and Zilberg2014). Egg production was defined as the mean number of eggs laid per parasite per day. Parasites were considered to be detached when they were completely separated from the surface of the culture vessel.
Due to the large number of adult parasites required, each treatment (plant extract and ethanol 70%) was evaluated in individual, staggered experiments, with a seawater control made for each experiment. Statistical comparisons were carried out independently for each treatment vs. their individual seawater control. All treatments and controls had six replicate wells, with five parasites per replicate well. Culture vessels were incubated at room temperature (26 ± 2°C) with a natural photoperiod. Parasites were monitored under a stereomicroscope every hour throughout an 8-h period. A final observation was then made 24 h after immersion.
Egg development and hatching success
Newly laid parasite eggs were collected from the laboratory infection to examine development and hatching success in the plant extracts and ethanol. Five fish were moved to a clean tank and eggs were collected over a 24-h period using clean nylon threads. The threads were cut using fine dissecting scissors under a stereomicroscope into ~1-cm segments with 10 eggs each and transferred to six-well flat-bottom culture vessels (15.5 ml; Becton Dickinson Labware) containing the treatment solutions. All treatments, ethanol (1:10, 1:50 and 1:100), plant extracts (1:10, 1:50 and 1:100) and seawater control were tested with nine replicates spread across two plates, with each replicate containing 10 eggs. Eggs were exposed to continuous immersion in each treatment and control for 9 days, incubated at room temperature 26 ± 2°C under natural photoperiod. Every 24 h, eggs were observed under a stereomicroscope and development was scored following Hutson et al. (Reference Hutson, Mata, Paul and de Nys2012) (stage I = non-embryonated; stage II = embryonated; stage III = developing and stage IV = hatched). Hatching success (%) was calculated based on the number of eggs with an opened operculum.
Oncomiracidia longevity
Newly laid eggs (<24 h old) from the laboratory infection were collected and incubated under laboratory conditions for 7 days in a Petri dish with filtered seawater at room temperature (26 ± 2°C) and natural photoperiod. On day 6 of incubation the Petri dish was covered with aluminium foil for complete darkness; on day 7 the Petri dish was uncovered and exposed to light to stimulate hatching (Hoai & Hutson, Reference Hoai and Hutson2014).
Oncomiracidia were incubated in 96-well microplates (Greiner Bio-One, Stonehouse, Gloucestershire, UK). For each plant extract, ethanol treatment and seawater control, a single oncomiracidium (5 ± 1 h old) was individually pipetted into each well, with 36 replicates used per treatment. Oncomiracidia were exposed to 8 h continuous immersion in each treatment at room temperature (26 ± 2°C). Wells were monitored under a stereomicroscope every hour following immersion, for eight consecutive hours. Time of death was determined when larvae stopped moving and failed to respond to a light stimulus (Fridman et al., Reference Fridman, Sinai and Zilberg2014).
Statistical analysis
Results for each parameter were expressed as an arithmetic mean ± SE. Data were log10 transformed to satisfy normality and homogeneity of variance requirements. A general linear model (analysis of variance (ANOVA)) was used to detect significant differences between each treatment and the corresponding control. Post-hoc comparisons were made via Tukey's HSD tests and statistical significance was accepted at P < 0.05. The statistical analyses were performed in R v. 3.1.3 (R Core Team, 2015).
Results
Adult parasite survival, egg production and time to detachment
In garlic extract, parasite survival was not significantly reduced. Ginger and basil extracts both compromised adult parasite survival in vitro. Adult parasite survival was significantly reduced when treated with all dilutions of ginger extract compared to seawater control. Basil 1:100 and 1:50 significantly reduced adult parasite survival (Tukey, P = 0.002 and P = 0.0001, respectively). When immersed in basil, parasites survived 12.80 ± 1.98 h (1:100) and 10.10 ± 1.59 h (1:50) compared to the seawater control where parasites survived for almost twice as long, 23.36 ± 0.64 h (table 1). There was no effect of the bitter chaparro, onion or papaya extracts on adult parasite survival, and throughout the trial parasites remained transparent and attached to the surface of the culture vessel (table 1). In the ethanol only treatment, dilution 1:10, significantly reduced (P = 0.0005) survival to 18.66 h compared to the seawater control (24 h).
Table 1. Neobenedenia sp. adult survival, mean egg production and time for parasites to detach from culture vessel, during 24-h immersion in plant extracts, ethanol treatment and seawater (35 g l−1).

Data expressed as arithmetic mean ± standard error (SE).
* Values with statistical difference when compared to control (P < 0.05), shown in bold type.
Garlic treatments did not affect mean egg production when compared to the seawater control (table 1). However, mean egg production was significantly reduced in the three dilutions of ginger (1:100 dilution P = 0.00008, 1:50 dilution P = 0 and 1:10 dilution P = 0), basil (1:100 dilution P = 0.00002, 1:50 dilution P = 0.000003 and 1:10 dilution P = 0.0) and bitter chaparro (1:100 dilution P = 0.00005, 1:50 dilution P = 0.000004 and 1:10 dilution P = 0.0). Indeed, in these treatments egg production was reduced by more than 70% compared to seawater controls (table 1). In treatments with onion and papaya, only high concentrations of extracts (1:10) significantly reduced the mean egg production of parasites (P = 0.0 and P = 0.0, respectively). Adults immersed in ethanol 1:50 and 1:10 dilutions showed significantly reduced egg production, to 3.2 ± 1.33 and 0.43 ± 0.23, respectively, compared to 39.56 ± 4.71 mean egg production in the seawater control (table 1).
Early detachment of the parasites from the surface of the culture vessel was observed in all three dilutions of ginger (1:10, 1:50 and 1:100). Ginger extract exhibited the most toxic effect on adult parasites; we observed a change of body colour from transparent to opaque and contracted opistohaptors. In the 1:10 dilution of ginger, the time taken for parasites to detach from the surface of culture vessel was reduced to 1.56 ± 0.24 h, compared to the seawater control (10.80 ± 1.51 h) (table 1). Basil dilutions 1:100 and 1:50 significantly reduced (P = 0.003 and P = 0.00005, respectively) the time for parasites to detach from the surface compared to the seawater control (table 1). In the garlic and onion extracts, the time to parasite detachment was significantly reduced only in the 1:10 dilution (P = 0.00007 and P = 0.002, respectively). There was no effect of bitter chaparro or papaya extracts on time to parasite detachment (table 1). In ethanol treatments, the 1:50 and 1:10 dilutions significantly reduced the mean time for parasites to detach (P = 0.0001 and P = 0.0, respectively).
Egg development and hatching success
There was no egg development in any of the plant extracts or ethanol at 1:10 dilutions; eggs remained clear brown in colour during the 8 days of immersion, with 0% hatching success (fig. 2c). Hatching success was significantly reduced in basil extract (1:100) to 86.6% and in ethanol (1:50) to 71.11%, compared to all other plant extracts, ethanol treatments and the seawater control, where 100% hatching success was obtained (fig. 2a). There was no significant impact on hatching success in any of the plant extracts examined at 1:50 dilution (fig. 2b) and eggs in all treatments exhibited 100% hatching success. In dilutions 1:50 and 1:100 of all plant extracts, and seawater controls, eggs began hatching on day 5 and finished on day 8. In ethanol treatment 1:50 and 1:100 hatching was delayed until day 7 and finished on day 8. In the first 3 days following collection, the eggs changed from clear to dark brown with a granular appearance, indicating cell division. On day 4, embryos started to exhibit eyespots. Eggs started hatching on day 5 and ceased on day 8.
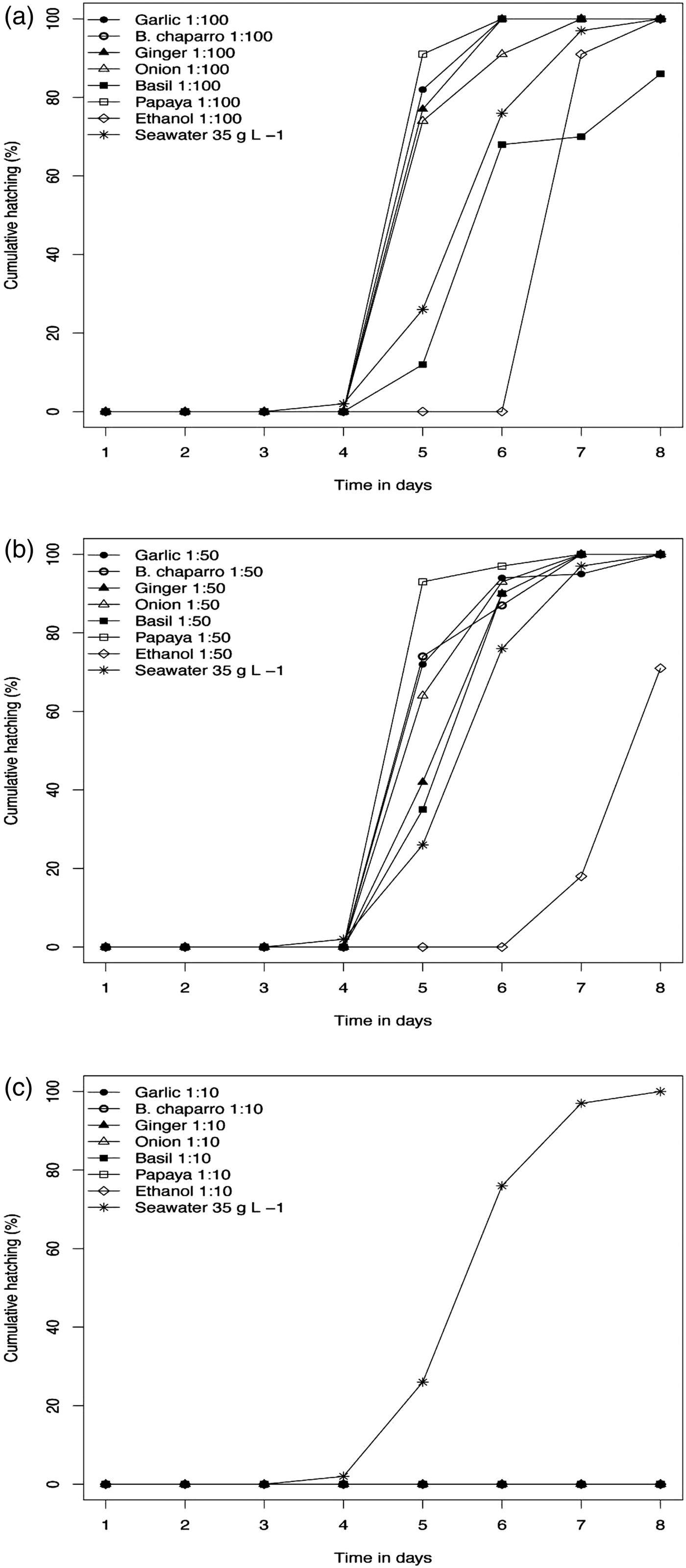
Fig. 2. Cumulative hatching (%) of Neobenedenia sp. eggs exposed to plant extracts, ethanol treatments and seawater control. (a) 1:100 dilution, (b) 1:50 dilution, and (c) 1:10 dilution.
Oncomiracidia longevity
With garlic extract, only the 1:10 dilution significantly reduced the longevity of oncomiracidia (P = 0.0005). However, all dilutions of ginger and basil extracts reduced the longevity of oncomiracidia. Ginger extract killed all oncomiracidia in less than 4 h, compared to the seawater control where oncomiracidia exhibited a mean life span of 7.47 ± 0.29 h (table 2). Longevity was significantly reduced in 1:50 and 1:10 dilutions of bitter chaparro. No reduction in larvae longevity was obtained when parasites were immersed in onion and papaya treatments compared to seawater controls (table 2). We observed a significant reduction in the longevity of oncomiracidia when immersed in ethanol 1:50 (P = 0.0013) and 1:10 (P = 0).
Table 2. Survival of Neobenedenia sp. oncomiracidia, during 8-h immersion in plant extracts, ethanol treatment and seawater (35 g l−1).

Data expressed as arithmetic mean ± standard error (SE).
* Values with statistical difference when compared with each control (P < 0.05), shown in bold type.
Discussion
Ginger (Z. officinale) is a medicinal plant widely used for treatment of infectious diseases and helminthiasis (El-Bahy & Bazh, Reference El-Bahy and Bazh2015). The main active components are gingerols, shogaol and curcumin (Lin et al., Reference Lin, Chen, Lu, Ma, Chung, Wang, Lee and Yen2014). In our study, immersion in ginger extract reduced adult and oncomiracidia survival. Adults quickly contracted and detached from the surface of the culture vessel, suggesting damage to attachment structures, such as anterior attachment organs, posterior opistohaptor and muscular elements. Therefore the ability of the parasite to reproduce and subsequent egg production were significantly reduced. The anthelminthic properties of ginger have been proven in in vitro and in vivo studies with the monogenean G. turnbulli infecting the guppy P. reticulata (Levy et al., Reference Levy, Zilberg, Paladini and Fridman2015), terrestrial parasites Shistosoma mansoni (Sanderson et al., Reference Sanderson, Bartlett and Whitfield2002; Mostafa et al., Reference Mostafa, Eid and Adly2011), Raillietina cesticillus (El-Bahy & Bazh, Reference El-Bahy and Bazh2015) and gastrointestinal nematodes of sheep (Iqbal et al., Reference Iqbal, Lateef, Akhtar, Ghayur and Gilani2006), among others. Bioactive components [10]-shogaol, [6]-gingerol, [10]-gingerol, [6]-shogaol, gingerenone A, [6]-dehydrogingerdione, [4]-shogaol, 5-hydroxy-[6]-gingerol, hexahydrocurcumin, 3R, 5S-[6]-gingerdiol and 3S,5S-[6]-gingerdiol isolated from ginger have been proven responsible for reducing movement and survival of larvae of the nematode Angiostrongylus cantonensis and the cestode Hymenolepis nana (Lin et al., Reference Lin, Chen, Chung and Yen2010, Reference Lin, Chen, Lu, Ma, Chung, Wang, Lee and Yen2014).
In folk medicine, basil leaves are used for carminative, stomachic, antispasmodic, stimulant, diuretic, antiseptic, anaesthetic, analgesic and anthelmintic purposes, among others (Shirazi et al., Reference Shirazi, Gholami, Kavoosi, Rowshan and Tafsiry2014). In aquaculture, basil has been evaluated against bacterial infections of Aeromonas hydrophila in Oreochromis mossambicus (Bulfon et al., Reference Bulfon, Volpatti and Galeotti2015). In Penaeus (shrimp) larviculture, it is reported to have characteristics of growth promotion, immunostimulation and antibacterial activity (Citarasu, Reference Citarasu2010). The primary bioactive components of basil are phenoyl derivatives, such as eugenol, methyleugenol, chavicol, estragole and methyl-cinamate, often combined with various amounts of linalool (Filip et al., Reference Filip, Vidović, Vladić, Pavlić, Adamović and Zeković2016). The antiparasitic effect of basil extract has been related to the linalool content. De Almeida et al. (Reference De Almeida, Alviano, Vieira, Alves, Blank, Lopes, Alviano and Rosa2007) reported that linalool contained in basil (O. basilicum) inhibits the proteolytic activity of peptidases and they suggested that, through this mechanism, basil extract inhibits enzymatic processes, leading to damage and death of parasites. In our results, 1:100 and 1:50 dilutions showed a significant reduction in survival, egg production, time to detachment of adults and survival of oncomiracida. However, in the 1:10 dilution the parasites remained alive (responding to tactile or light stimuli) during the experiment, yet no eggs were produced. It is unknown if this is the result of the generalized cytotoxic damage or of a specific inhibition of the reproductive processes by the compounds present in the basil extract. Sutili et al. (Reference Sutili, Murari, Silva, Gressler, Heinzmann, Vargas, Schmidth and Baldisserotto2016) evaluated essential oil of basil (O. americanum) against the monogenean Gyrodactylus sp. They reported that exposure to 50 mg l−1 of basil reduced survival in vitro and in vivo, leading to a significant reduction in the number of parasites infecting silver catfish (Rhamdia quelen).
Bitter chaparro (C. tortuosa) extract has been used previously for the treatment of bacterial, amoebic and parasitic infections (Robles-Zepeda et al., Reference Robles-Zepeda, Velázquez-Contreras, Garibay-Escobar, Gálvez-Ruiz and Ruiz-Bustos2011). In the present study bitter chaparro extract reduced egg production of Neobenedenia sp. (table 1) and reduced survival of oncomiracidia (table 2). The toxic effect of this plant has been attributed to secondary metabolites. The main biocomponent identified with antiparasitic properties is chaparrin ((Reyes-López et al., Reference Reyes-López, Villa-Treviño, Arriaga-Alba, Alemán-Lazarini, Rodríguez-Mendiola, Arias-Castro, Fattel-Fazenada and de la Garza2005; Aguilar et al., Reference Aguilar, de Gives, Sánchez, López-Arellano, Hernández, Aroche and Valladares-Cisneros2008).
Continuous immersion in garlic, onion or papaya extract at 1:100 and 1:50 dilutions did not impact any of the life stages of Neobenedenia sp. (tables 1 and 2, fig. 2a, b). However, as with most of the plant extracts, the 1:10 dilutions were more effective when compared to the seawater control. Garlic and onion extracts have been reported to be vermifuges and insect repellents (Guarrera, Reference Guarrera1999) and are constituents of anthelmintic remedies for animals and humans (Melhorn et al., Reference Melhorn, Wu and Ye2013). Their incorporation in shrimp food is recommended to prevent bacterial infections (Citarasu, Reference Citarasu2010). Garlic-based treatments have been shown to prevent parasitic infections in tilapia fry, O. niloticus, in barramundi, L. calcarifer and guppy, P. reticulata (Abd El-Galil & Aboelhadid, Reference Abd El-Galil and Aboelhadid2012; Militz et al., Reference Militz, Southgate, Carton and Hutson2013a, Reference Militz, Southgate, Carton and Hutsonb; Fridman et al., Reference Fridman, Sinai and Zilberg2014; Levy et al., Reference Levy, Zilberg, Paladini and Fridman2015). Both plants are composed mainly of water and the most significant components are the organosulphur-containing compounds, such as alliin and allicin, and also, in onion, flavonoids (Benkeblia, Reference Benkeblia2004). The antiparasitic activity of these vegetables is attributed primarily to the content of allicin; however, this compound is unstable and processing methods greatly affect the chemical structure of garlic preparations, thereby affecting the antiparasitic activity of these plant extracts (Corzo-Martínez et al., Reference Corzo-Martínez, Corzo and Villamiel2007; Lee & Gao, Reference Lee and Gao2012). Antiparasitic properties of papaya have been reported for the treatment of cestode and nematode infestations in mice (Abou Shady et al., Reference Abou Shady, Basyoni, Mahdy and Bocktor2014), Ichthyophthirius multifiliis parasite infections in goldfish Carassius auratus (Ekanem et al., Reference Ekanem, Obiekezie, Kloas and Knopf2004) and intestinal parasites in humans (Alanís et al., Reference Alanís, Calzada, Cervantes, Torres and Ceballos2005). The medicinal properties of papaya are well documented, and each part of the plant contains different enzymes and compounds of interest (Vij & Prashar, Reference Vij and Prashar2015). Anthelmintic activity is based on the compound benzyl isothiocyanate obtained mainly from papaya seeds (Kermanshai et al., Reference Kermanshai, McCarry, Rosenfeld, Summers, Weretilnyk and Sorger2001). However, the water–ethanol extracts of garlic, onion and papaya evaluated in the present research did not show antiparasitic properties against Neobenedenia sp., most probably because of the instability or low concentration of bioactive compounds.
Monogenean eggs have been reported to have high resistance to external factors (Whittington, Reference Whittington, Woo and Buchmann2012) due to the proteinaceous shell that protects the developing embryo from chemical and physical agents. However, in 1:10 dilutions of all plant extracts and ethanol it was found that Neobenedenia sp. hatching did not occur and larval development failed within 8 days of continuous immersion. Previous reports have demonstrated that desiccation, immersion in water at 50°C, 25% ethanol (Ernst et al., Reference Ernst, Whittington, Corneillie and Talbot2005), 120 mg l−1 of sodium hypochlorite (Fajer-Ávila et al., Reference Fajer-Ávila, Velásquez-Medina and Betancourt-Lozano2007), hyposalinity (Chen et al., Reference Chen, Chen, Wang, Chen and Shih2010) and a water-soluble extract of red seaweed Asparagopsis taxiformis (Hutson et al., Reference Hutson, Mata, Paul and de Nys2012) are also effective against monogenean eggs.
An extraction process with ethanol is one of the most commonly used for traditional Chinese medicines (Van Hai, Reference Van Hai2015). In the present research, ethanol (70%) in different seawater dilutions (1:100, 1:50 and 1:10) impacted many parameters of the three life stages of Neobenedenia sp. Levy et al. (Reference Levy, Zilberg, Paladini and Fridman2015) compared the efficiency of an ethanolic extract and an aqueous extract of ginger, and ethanol 75% against the monogenean parasite G. turnbulli, and also reported that exposure to ethanol 75% affected survival in vitro of G. turnbulli; however, when comparing efficacies between ethanolic and aqueous ginger extracts, the ethanolic extract was found to be much more efficient. These authors suggested that the result was due to the fact that the majority of lipophilic compounds that affect the parasite were obtained by ethanol extraction. The compounds of an ethanol extract of the Chinese herb Arisaema erubescens were found to be two times more toxic against the plant parasite Meloidogyne incognita than those of the crude extract (Du et al., Reference Du, Zhang, Bai, Wang, Liu, Liu, Wang and Deng2011). In general, when extraction solvents are compared, alcoholic solvents provide a higher efficiency in extracting secondary bioactive metabolites compared to water-based methods (Bulfon et al., Reference Bulfon, Volpatti and Galeotti2015).
Biological parameters of Neobenedenia sp. were most negatively impacted with the 1:10 dilutions of ginger and basil. Our study demonstrates that a synergistic effect of ethanol and bioactive components in ginger, basil and bitter chaparro extracts is toxic against Neobenedenia sp. life stages. Ginger and basil extracts reduced adult survival in vitro, time to detachment from the surface of the culture vessel, egg production and oncomiracidia survival. Bitter chaparro extract reduced adult egg production and oncomiracidia survival.
In conclusion, this study demonstrates the potential use of plant extracts for the treatment of Neobenedenia sp. infections. Of the six plant extracts, ginger and basil proved to be most effective against adults and oncomiracidia of Neobenedenia sp. Both show potential for alternative methods to manage parasitic disease in aquaculture.
In future experiments, controlled amounts of isolated active biocomponents should be assessed to allow estimation of the effectiveness of each bioactive product. Such studies will greatly contribute to the design of practical solutions to reduce parasite burdens in aquaculture.
Acknowledgements
We thank Elisa Trasviña Nuñez, Guadalupe A. Javalera Manriquez and Enrique Calvillo Avilés for laboratory assistance and Raul Martínez Rincon for statistical advice. We are grateful to the Baja Seas fish farm in Bahía Magdalena, BCS for providing juvenile fish.
Financial support
Funding for this project was provided by the Consejo Nacional de Ciencia y Tecnología (CONACYT, CB-155381) and CIBNOR (PAC). A.G.T.M. is a recipient of a doctoral fellowship from CONACYT (164850).
Conflict of interest
None.