Traditional foods (TF) are edible species that belong to a traditional food system, whether in developed( Reference Galli, Cavicci and Santini1 ) or developing countries( Reference Kuhnlein and Receveur2 ). In developing countries, the term TF is often associated with traditional food systems of indigenous people which include foods from local and natural environments that are culturally acceptable( Reference Kuhnlein and Receveur2 ). Importantly, localization is used to emphasize the boundaries and differentiation of a culturally homogeneous community( Reference Hinrichs3 ). Research on TF is emerging because of social, conservation and economic challenges( Reference Galli, Cavicci and Santini1 ).
Malnutrition in developing countries is a long-lasting burden, despite their diverse agricultural production and availability of wild foods( Reference Vinceti, Termote and Ickowitz4 ). Availability of a diversity of foods seems to be a necessary, but not a sufficient, condition to reach nutrient security( Reference Termote, Bwama Meyi and Dhed’a5 ). Although there is not much evidence to affirm that TF consumption supplies enough dietary energy, it covers a high share of the micronutrient requirement, and therefore TF are seen as important elements of the food system to fight hidden hunger( Reference Penafiel, Lachat and Espinel6 , Reference Powell, Thilsted and Ickowitz7 ). It is currently understood that consumers who have access to self-cultivated foods and foods from wild sources have a dietary diversity which is rich in species( Reference Kennedy, Stoian and Hunter8 ). Recent research indicates that species richness in the diet of rural women is an adequate indicator for nutrient adequacy in developing countries( Reference Lachat, Raneri and Smith9 ).
More specifically, studies have shown that eating a variety of local fish species in poor rural communities of Bangladesh provides consumers with 31–40 % of the recommended intake of vitamin A and Ca, respectively( Reference Roos, Islam and Thilsted10 ). In rural villages in Vietnam, eating wild vegetables contributes 21 % of folate intake( Reference Ogle, Johansson and Tuyet11 ). The consumption of a high variety of local Arctic foods by local Canadians provides them with 87, 23 and 37 % of their total daily intake of cholecalciferol, vitamin A and tocopherol, respectively( Reference Kuhnlein, Barthet and Farren12 ). High agricultural biodiversity, cultivated and wild food species, is correlated with high nutrient adequacy ratios for Zn, vitamins B12, B6 and C, folate and riboflavin in two rural Kenyan villages( Reference M’Kaibi, Steyn and Ochola13 ).
Undoubtedly, the study of TF includes the intake of both locally cultivated and wild foods. In rural areas, diets are often diversified by plant and animal foods obtained from agricultural production( Reference M’Kaibi, Steyn and Ochola13 – Reference Powell, Hall and Johns17 ), wild foods from the forest( Reference M’Kaibi, Steyn and Ochola13 , Reference Powell, Hall and Johns17 – Reference Passos, Mergler and Fillion21 ), and wild freshwater fish or fish from aquaculture( Reference Roos, Islam and Thilsted10 , Reference Roos, Thorseng and Chamnan22 – Reference Bogard, Thilsted and Marks24 ). However, cultivated and wild TF can coexist in farms (i.e. wild vegetables found around cultivated areas or home gardens); whereas forests are mainly the source of wild TF (i.e. wild fruits and bush meat).
Despite the increasing body of evidence about the nutrient contribution of certain species, the role of a variety of TF in nutrition is still not solid. Nutrition interventions in developing countries require that the nutrient contribution of TF, wild or cultivated, is documented prior to the design of local strategies such as promoting TF consumption or policy development. Ecuador is one of the seventeen megadiverse countries in the world in which research is required to document the nutrient contribution of a diet composed of a variety of TF.
Methods
The present research documented all edible items present in the rainy season using a food intake study. For TF, the analysis included only foods that were: (i) locally cultivated or found in the wild at Guasaganda (geographical location); (ii) recorded during the food intake study (dietary surveys); (iii) collected in situ (species identification); and (iv) cooked locally (recipe preparation).
Study area and participants
Guasaganda is a tropical rural parish located in central Ecuador in the Andes region with altitudes that vary between 250 and 1000 m above sea level. This geographical area was selected because it is a centre of food biodiversity( Reference Aguilar and Cartagena25 ). The population (about 3900 inhabitants) is mainly indigenous( 26 ). Rural adult women of indigenous ethnicity were selected to be the study participants because they are vulnerable to overweight and micronutrient deficiencies and are the poorest segment of the population( Reference Freire, Ramírez and Belmont27 ). The data collection was conducted during the rainy season assuming that is the highest peak of food biodiversity( Reference M’Kaibi, Steyn and Ochola13 ).
Dietary surveys
Recording the consumption of foods is a methodology widely used to assess the nutrient intake and dietary diversity of populations( Reference Salvador Castell, Serra-Majem and Ribas-Barba28 ). One of the most commonly used approaches is the 24 h recall since it is very practical, has a relatively low cost and can be administered by trained interviewers( Reference Salvador Castell, Serra-Majem and Ribas-Barba28 , Reference Fiedler, Martin-Prevel and Moursi29 ). The present survey was conducted between March and May 2011 and started with a 24 h recall questionnaire which was repeated after 14 d in a second visit. Two non-consecutive visits can help to reduce intra-personal variability and to further compute usual food intakes( Reference Otten, Hellwig and Meyers30 ). The studied individuals were adult rural women (older than 18 years) living in Guasaganda who were neither pregnant nor breast-feeding and were residents of Guasaganda for more than a year. Women were interviewed because they are responsible for food preparation in rural areas.
The questionnaire recalled all food eaten in the previous 24 h, including the amount of food consumed (using standardized measuring tools), the local name of each food used to prepare a recipe with its food source (i.e. collected/hunted or cultivated, purchased but not processed, purchased or processed food, food aid), brands of commercialized foods and cooking method (i.e. raw, cooked, fried, oven). Also, demographic information documented age, occupation and education level (more details on the levels used for occupation and education can be found in Table 1). Interviews were administered to participants at their house and in Spanish, by trained interviewers supervised by the main author (D.P.) who speaks the local language. Interviews conducted by the author served as control performance for the other interviewers. Protocols were developed using literature on dietary surveys( Reference Willett31 , Reference Gibson32 ) and food biodiversity manuals( 33 ).
Table 1 Demographic characteristics of the studied rural women (n 178) and their respective partners from Guasaganda, Ecuador

The number of women to be interviewed was calculated by using G*Power software (general power analysis) version 2009, aiming for a precision of 0·5 sd and 80 % power based on the mean Traditional Food Diversity Score (TFDS; 9·5 (sd 3·5)) from a study in Peruvian indigenous people( Reference Roche, Creed-Kanashiro and Tuesta34 ), generating 128 individuals. We multiplied the latter number by 1·2 assuming the villages as clusters. In addition, twenty-four households (15 %) were added for contingency considering that in rural areas some households may drop out of the study. The final calculated sample size was 178.
Sampling was done following the recommendation from the Food and Nutrition Technical Assistance Project manuals( Reference Magnani35 , Reference Swindale and Ohri-Vachaspati36 ) to use a two-step cluster design. In the first step, villages were selected from a list of all villages in the parish of Guasaganda retrieved from the Ecuadorian Institute of National Surveys using the probability proportional to size method. The second step involved a random selection of households within each village using a map created by us that positioned each house using GPS (Global Positioning Satellite) points. By this method, ten villages were selected to be studied out of the thirty-eight. In each household, one woman in charge of TF preparation was interviewed. In each visit, a kit containing a standardized cup, dish and spoon were provided by the interviewer to the participant, aiming for more accuracy when describing portion sizes.
A third visit to the villages (June 2012) was done to record the frequency of consumption of twenty local foods using an FFQ querying frequencies of consumption in times per day, week and month. The consumption frequency is essential to calculate usual intakes with the Multiple Source Method (MSM) using MSM software version 1.0.1 (https://msm.dife.de/).
Traditional food identification
Only edible species found in the area were identified as TF. A local guide assisted us to find the wild plant species with edible use in the forest of Sacha Wiwua (Central Guasaganda) and crops in surrounding farms. We collected all plants with edible use in 2012 (from June until August) following the methodology described in the literature( Reference Bonham37 , Reference Elzinga, Salzer and Willoughby38 ). The Ecuadorian Ministry of Environment provided a scientific permit allowing the collection of edible plant samples (03-12IC-FAU-FLO-OPAC/MA). Animal samples were not collected because this was not allowed by the Ministry of Environment, aiming to preserve local animal species. Scientific names of plants were given following the botanical binomial nomenclature. For the latter, we used the official names as registered in a web catalogue (www.theplantlist.org, version 1.1). The scientific names for local birds, mammals and fish were retrieved from three web catalogues of Ecuador( Reference Freile and Bonaccorso39 – Reference Burneo and Boada41 ).
Recipe preparation
The present study recorded traditional recipes to document the amount of each ingredient per total weight for the final dish (e.g. chicken soup with plantain). We prepared traditional recipes at least three times to correct for different cooking recipes. Preparation of the latter was done using local ingredients, in local kitchens and using local utensils. Each ingredient was weighed using a digital scale (± 0·01 g) which measured the ingredients in grams to two decimal places (Mettler Toledo, EL 2001). The mixed method for recipe calculation recommended by the FAO was used to correct nutrients from a food composition table (FCT) at ingredient level by using nutrient retention factors( 42 ) and yield factors( Reference Berstronm43 ) according to the preparation method reported in the 24 h recall. By this method, the percentage of weight change, or the percentage of nutrient retention or loss, due to cooking was corrected for. For example, when plantain is cooked in a soup it tends to absorb water and increase its weight but also loses vitamin C due to heating. By this method, we could calculate the final weight and vitamin C content by correcting the values obtained from the FCT for plantain in the raw state.
Analysis
For the statistical analysis, data from the first visit included 178 sampled women and for the second visit 167. To simulate the usual diet the third visit included only 127 women.
Descriptive statistics were performed in R software (2011) to get insight into the demographic characteristics (see Table 1) and the usual diet of the studied group. All food intake data from the 24 h recalls were managed in Lucille application version 0.1.6.8. The latter exported an Excel document with the list of all consumed foods, the quantified intake in grams per person and nine nutrient intakes per person per day. This Excel data set (available at doi:10.6084/m9.figshare.7851584) was used for further analysis in R, after handling missing data and outliers.
Lucille merged the intake in grams with the nutrient content of foods from a compiled FCT following a standard methodology( Reference Charrondiere and Burlingame44 ). We used the FCT of Peru( 45 ) and Central America( 46 ) assuming that these are similar geographical areas which supply foods with comparable nutritional content (each nutrient per 100 g). The FCT of Ecuador was used only for foods that were not found in the other two FCT because it is a very old publication( 47 ). The US Department of Agriculture’s National Nutrient Database for Standard Reference was the resource for missing nutrients( 48 ). The studied nutrients included protein, fat, carbohydrates, fibre, Ca, Fe, Zn, vitamin A and vitamin C. Vitamin D was not studied since in Ecuador this vitamin is mainly synthesized in the skin under high sun exposure( 49 ). Iodine was also not considered because 99 % of the Ecuadorian population consumes iodized salt( 50 ). We calculated total energy intake in kilojoules using Atwater factors for macronutrients because under-reporting of energy intake often occurs when using 24 h recalls( Reference Johansson, Wikman and Ahren51 ). The results report the central tendency by use of the median, the minimum and maximum.
To report the diversity of the diet, Dietary Species Richness (DSR; the total number of species) and TFDS (the number of locally produced and wild species) were reported using the methodology of Lachat et al.( Reference Lachat, Raneri and Smith9 ) and Roche et al.( Reference Roche, Creed-Kanashiro and Tuesta34 ), respectively. In addition to dietary diversity, to analyse the quality of the diet, the Minimum Dietary Diversity for Women of reproductive age (MDD-W) was calculated. Foods were grouped into ten food groups as proposed in the literature( 52 ). MDD-W is a dichotomous dietary quality indicator that shows micronutrient adequacy of the diet when more than five food groups (out of the ten) are consumed and has been validated for developing countries( 53 ). The food groups included: (i) all starchy and staple foods; (ii) beans and peas; (iii) nuts and seeds; (iv) dairy; (v) flesh foods; (vi) eggs; (vii) vitamin A-rich vegetables and fruits; (viii) other fruits; (ix) vitamin A-rich dark green vegetables; and (x) other vegetables (analysis with MSM software version 1.0.1 excluded the last two because these were not consumed by any respondent).
The nutrient intake data were imputed for missing data and then analysed using R. For those women who were not at home at the second visit (n 11), missing data imputation was conducted using multivariate imputation by chained equations (MICE) by the second researcher (H.C.-V.) using the R package mice( Reference Van Buuren and Groothuis-Oudshoorn54 ). The latter used ten imputations per missing datum, and the mean was used to replace the missing value. MICE draws from the theoretical joint posterior distribution of the missing data by specifying for each incomplete variable a conditional model for the missing data given a set of other variables (fully conditional specification). Starting from an initial imputation, MICE draws imputations by integrating over the conditional densities. By modelling only conditional distributions, many complexities of real multivariate data such as predictors of a different type, the existence of non-linear relationships or interactions between variables and circular dependence can be addressed( Reference Van Buuren and Groothuis-Oudshoorn54 , Reference Van Buuren55 ). For the present study, MICE imputed each incomplete nutrient by generating plausible synthetic values given the other nutrients in the data. In R, we used the default values of the R mice(·) function with predictive mean matching for the conditional models. MICE has been used in medical research( Reference Van Buuren56 ). Various authors have shown the satisfactory performance of this method( Reference Van Buuren, Brand and Groothuis-Oudshoorn57 – Reference Hapfelmeier, Hothorn and Ulm59 ).
Because we did not find statistical evidence of normality for the nutrient data as revealed by Quantile–Quantile plots and Kolmogorov–Smirnov tests (results not shown), we reported the first quartile, median and third quartile for nutrient intakes (see Table 2). Reporting these quartiles and the median aims to summarize the diet composition data and eases the interpretation for non-scientist readers( Reference Lang and Secic60 ). Note that these measures cannot be summed up to obtain the composition per 100 g, nor for nutrients representing sources of energy (proteins, carbohydrates, fibres, fats), when applying the Atwater equation to estimate kilojoules.
Table 2 Descriptive statistics of women’s diet in Guasaganda, Ecuador, during the rainy season of 2011 (n 178), the percentage of women below the Estimated Average Requirement (EAR) for the listed nutrients and the Nutrient Adequacy Ratio (NAR)
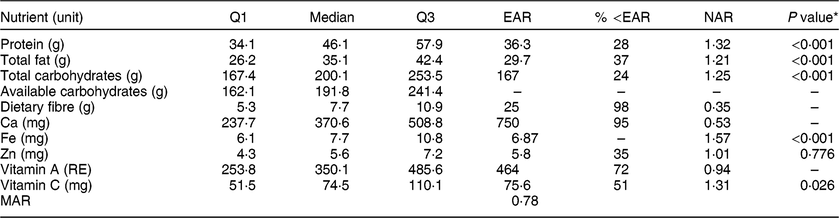
Q1, first quartile; Q3, third quartile; RE, retinol equivalents; MAR, Mean Adequacy Ratio.
The EAR for available carbohydrates was not used.
The EAR cut-off method was not used for Fe as it does not meet the conditions for this methodology(Reference Otten, Hellwig and Meyers30).
Q1, median and Q3 cannot be summed up to obtain the composition per 100 g, nor for estimation of energy intake using Atwater factors.
* Wilcoxon rank test at the 5 % significance level to test if the intake was significantly higher than the EAR for nutrients that reported a NAR greater than 1.
The Nutrient Adequacy Ratio (NAR) was used as a measure of nutrient adequacy. NAR is obtained by dividing the quantified intake of a nutrient by the Estimated Average Requirement (EAR), as proposed in the literature( Reference Otten, Hellwig and Meyers30 , Reference Murphy61 ). The EAR is the intake sufficient to meet 50 % of the nutrient requirements (which in a normal distribution is the mean or median). Therefore, a person who consumes as much as the EAR for a specific nutrient has 50 % probability to meet the nutrient requirement. In this line, the EAR was used as the cut-off point. Table 2 shows the percentage of the population below the EAR (except for Fe because its distribution is known to be skewed)( Reference Murphy, Munro and Young62 ). Values below the EAR show that interventions are needed as the probability of adequacy is less than 50 %( Reference Murphy, Munro and Young62 ). Calculating NAR allows us to easily assess if the requirements are met. Values closer to 1 indicate that the intake is closer to the requirements. The EAR for protein was 0·66 g/kg assuming body weight of 55 kg and quality and digestibility of the protein as for eggs( 63 , 64 ). The requirement for fat intake was converted from 20 % of total energy consumption to grams of fat using the Atwater factor of 37·656 kJ/g( 65 ). The requirement for carbohydrate intake (50 % of the total energy intake) and fibre was for non-pregnant and not breast-feeding female adults( 66 , 67 ). The Ca requirement covered dermal and urinary losses( 68 , 69 ). The Fe requirement covered menstruation losses and assumes body weight of 55 kg and 12 % bioavailability as the diet is composed of rice, fruits and animal protein( 68 , 70 ). For the calculation of Zn requirement, we used a body weight of 55 kg and a bioavailability of 30 % since local diets consist of both animal and plant foods( 68 ). The EAR for vitamin A was 8·4 µg/kg and assumed a body weight of 55 kg( 71 ). The EAR for vitamin C assumed an absorption of 80 % and a body weight of 55 kg( 72 ). In order to obtain robust results to non-normal data, we used the non-parametric Wilcoxon signed-rank test to test if the intake was significantly higher than the EAR for nutrients that reported a NAR greater than 1( Reference Otten, Hellwig and Meyers30 , Reference Murphy61 ).
In the same line, linear quantile mixed models( Reference Geraci and Bottai73 ) were fitted to find whether there is a significant association between DSR and Mean Adequacy Ratio (MAR; see Table 3). The latter is a measure of the adequacy of the diet, which is the average of all NAR (truncated to 1). Similarly, we used linear quantile mixed models to investigate the association between consumption of local species and MAR. To adjust for potential confounding, for both analyses we considered age, occupation and education as fixed effects and village as random effect. The Akaike information criterion was used to select the best model in each analysis. We used the function lqmm(·) in the R package lqmm( Reference Geraci74 ) to fit linear quantile mixed models. The argument ‘tau’ was set to 0·5 to fit models for the conditional median of MAR, while we used the default settings for the rest of the arguments of the lqmm(·) function. This implies the assumption of a diagonal variance–covariance matrix for the random effects.
Table 3 Estimates of the effect of total number of edible species consumed (Dietary Species Richness; DSR) and number of locally cultivated and wild species consumed (Traditional Food Diversity Score; TFDS) on the Mean Adequacy Ratio (MAR) of women’s diet in Guasaganda, Ecuador, during the rainy season of 2011 (n 178)
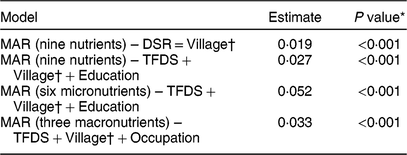
* The effect of DSR on MAR (nine nutrients) in the first model was tested at the 5 % significance level. The effect of local species (TFDS) on quality of the diet (MAR for all nine nutrients, for six micronutrients and for three macronutrients) was tested at the 1·66 % significance level by the Bonferroni correction.
† Best models shown above include a random intercept for village.
The median and interquartile range values of the ten most-consumed TF for the eight MDD-W food groups were calculated using the MSM (see Table 4). As mentioned above, locally cultivated vitamin A-rich dark green and other vegetables were not recalled during the interviews and therefore these were not reported in the results. The amount of each food consumed during the two-day record (first and second visit) and its consumption frequency (third visit) were imported into the MSM software version 1.0.1. This method applies two regression models, one for the positive daily intake data and one for the event of consumption, to simulate the usual diet. MSM estimates the individual probability of consuming a specific food or nutrient by means of a logistic regression model that used age and interviewer codes as covariates. Within-person variability was reduced by using the quantified intakes from the first visit and second visit, and using the frequency of consumption from the FFQ (third visit). The MSM uses the residuals of the regression to estimate variances and a Box–Cox transformation to estimate inter- and intra-individual variance.
Table 4 Usual intake* of the most-consumed traditional foods that contribute to the Minimum Dietary Diversity for Women (MDD-W) of women’s diet in Guasaganda, Ecuador, during the rainy season of 2011 (n 178)
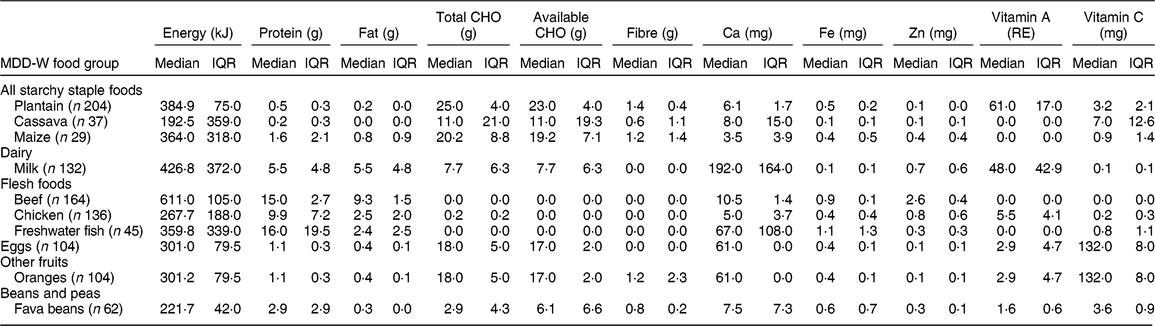
CHO, carbohydrates; RE, retinol equivalents; IQR, interquartile range; n, sample size.
* Usual intake computed using intakes from day 1 and day 2 and frequency of consumption, simulated by using the Multiple Source Method and MSM software version 1.0.1, only for food groups that were consumed in the two 24 h recalls.
The median intake of total fruits and of total vegetables was compared with the food intake recommendations for the prevention of chronic diseases( Reference Kromhout, Spaaij and de Goede75 ). Food-based dietary guidelines are often used to guide food and nutrition policies, health promotion interventions and disease prevention( 68 ). The non-parametric Wilcoxon signed-rank test was conducted to test for a significant difference between the median total fruit intake and the median total vegetable intake, and the recommended intake of 200 g fruits and 200 g vegetables, respectively, correcting for village. Because the recommendations of Kromhout( Reference Kromhout, Spaaij and de Goede75 ) (2016) are more recent than those of WHO( 76 ) (2003), we used the 200 g recommendation separately for fruits and vegetables rather than the 400 g merged. Also, the Wilcoxon signed-rank test was used to compare the median intake of ruminant meat with the recommendation of 50 g/d for sustainable diets, which is the maximum recommended to mitigate greenhouse gas emissions( Reference McMichael, Powles and Butler77 ).
Results
Table 1 shows the demographic characteristics of the rural respondents. The age of the studied women ranged between 18 and 69 years, with a median age of 39 years. Visited households had a maximum of 13 inhabitants and a median of 5. Most of the women practised family agriculture, whereas the minority were students. Regarding education, most of the studied women received only primary education.
The diet during the rainy season of 2011 was based on 140 different food items. The studied women consumed at least ten different foods per day. The median DSR was 16 (IQR 4), a minimum of 8 and maximum of 24, from a total of 119 species. During the period of data collection, seventy TF (meaning only locally cultivated and wild food species) were consumed. These included fifty plants species, thirteen animal species and seven medicinal plants (the list of species is available elsewhere(78)). The consumption of these TF led to a median TFDS of 6 (IQR 3), a minimum of 2 and a maximum of 14.
The median intake of protein, fat, total carbohydrates, Fe and vitamin C was each significantly higher than the EAR at the 5 % significance level, meaning that more of the required nutrients are consumed (NAR > 1). The MAR calculated for nine nutrients (truncated to 1) was 0·78, meaning that the diet is adequate for most of its nutrients (see Table 2).
The diversity of locally cultivated and wild foods provided 38·6 % of total energy intake. The latter excludes seven medicinal plants that do not contribute to energy because they are used as infusions.
The results of the linear quantile mixed models are shown in Table 3. Only the best models according to the Akaike information criterion are shown. For instance, the best model to investigate the association between the total number of species (DSR) and the quality of the diet (MAR for all nine nutrients) included our factor of interest, DSR, and a random intercept for village. We found a significant association between total number of total species (DSR) and quality of the diet (MAR for all nine nutrients, P < 0·001). Likewise, we found a significant association between local species (TFDS) and quality of the diet (MAR for all nine nutrients, for six micronutrients and for three macronutrients) at the 1·66 % significance level with the Bonferroni correction.
The studied women had a median MDD-W of 6 (with a minimum of 2 and a maximum of 8), out of 10 food groups, which is higher than the cut-off of 5, meaning that the studied diet is adequate in micronutrients( 53 ). Table 4 presents the usual intake of the top ten TF within MDD-W food groups.
Additional findings
The median fruit (91·73 g) and vegetable (25·56 g) intake was significantly lower than the 200 g for each food group, respectively, recommended for preventing chronic diseases( Reference Kromhout, Spaaij and de Goede75 ) (both P < 0·001). Analysis of ruminant meat intake showed that the median intake (63·86 g) was significantly higher than the maximum 50 g (P < 0·001) recommended for sustainable diets( Reference McMichael, Powles and Butler77 ).
An ingredient considered a condiment and used daily in almost every dish was coriander (Coriandrum sativum L.), which provided 4 % of the total vitamin A intake but was not counted as a vegetable within the MDD-W score.
Discussion
Results in the present study show a significant association between TF and MAR, after adjustment for education, village and occupation, during the rainy season of 2011. Results show that the studied diet is adequate for most of the studied nutrients, mainly micronutrients (MAR = 0·78). Dietary diversity is based on 140 different food items, from which half are locally cultivated and wild species. About six food species (TFDS) are consumed daily from local and wild sources. MDD-W of 6 shows the adequacy of micronutrient intake( 53 ). The most consumed TF, cultivated and wild, have been identified and quantified, which serves for future interventions that aim to promote TF consumption in the area.
Our study supports findings of similar studies done in rural communities in South America( Reference Roche, Creed-Kanashiro and Tuesta34 ), Canada( Reference Kuhnlein, Barthet and Farren12 ), Africa( Reference Termote, Bwama Meyi and Dhed’a5 ), Oceania( Reference Englberger, Aalbersberg and Schierle79 ) and South-East Asia( Reference Ogle, Hung and Tuyet19 ) in that there is statistical evidence to state that TF (whether cultivated or wild) contribute to a share of micronutrient intake. We may, however, have reported higher values than the referred studies because we merged cultivated and wild foods.
Our study differs from Lachat et al.( Reference Lachat, Raneri and Smith9 ) in that we counted only species which are locally produced (TFDS) and excluded foods that are not in the local environment. Our findings suggest that a local dietary species richness indicator can be useful for rural studies. According to Berti, the counting of food species has a high influence on the results and conclusions of the study( Reference Berti80 ).
Our results show that an increase in fruit consumption is required to reach the food intake recommendation (200 g/d) and prevent thereby chronic diseases( Reference Kromhout, Spaaij and de Goede75 ). We also suggest that the studied rural women need to consume a higher amount and diversity of vegetables. Consumption of locally cultivated and wild plant species that contribute to vitamin A intake is marginal. It is recommended to increase the cultivation of leafy vegetables or collect wild leafy vegetables to reach the 200 g recommendation( Reference Kromhout, Spaaij and de Goede75 ). Eating leafy vegetables has been reported to contribute to vitamin A, C and Ca intakes when 50 g/d are consumed( Reference Ogle, Hung and Tuyet19 ). The latter highlights the importance of consuming green leafy vegetables for micronutrient intake.
Ruminant meat consumption was reported as an additional finding regarding the sustainability of the diet. Results show that beef is the first local food source of energy. The population consumes significantly more than 50 g of ruminant meat daily which is the maximum recommended to mitigate greenhouse gas emissions( Reference McMichael, Powles and Butler77 ). Reducing the consumption of red meat to meet energy needs has shown to decrease diet-associated greenhouse gas emissions by up to 10·7 % ( Reference Vieux, Darmon and Touazi81 , Reference Auestad and Fulgoni82 ). Reducing the consumption of meat is, however, controversial because it supplies important nutrients such as Fe, Ca and vitamin B12 ( Reference Otten, Hellwig and Meyers30 ).
A novel approach of the present study is the use of MICE for the imputation of missing values. We suggest that the use of this method is adequate for rural studies based on previous literature( Reference Van Buuren, Brand and Groothuis-Oudshoorn57 – Reference Hapfelmeier, Hothorn and Ulm59 ). Often, rural studies face the limitation that participants drop out of the study, originating missing data during sampling. In this case, the second visit was not completed because a torrential rainfall limited the access to some households( Reference Mejia83 ). We recommend adding 15 % of households for contingency to have enough sample data.
Future studies on TF in Ecuador need to have access to more accurate and updated FCT( Reference Ramirez, Silva-Jaramillo and Belmont84 ). High-quality FCT should be representative of national foods including the habitual portions( 85 ). Nutrient content analysis of TF needs to be conducted by certified laboratories (e.g. ISO 17025) to report accurate and reliable FCT. This will allow nutritionists to identify micronutrient-rich foods that need to be promoted during healthy eating interventions. Prior to nutrient analysis, each species should be adequately identified, together with its cooking method and its geographical area such as the FCT of Guatemala( 85 ). For example, using a standardized sampling and analytical protocol on 171 genotypes of Musa spp. fruits from different geographical areas allowed identification of provitamin A-rich fruits( Reference Davey, Van den Bergh and Markham86 ).
Conclusion
Cultivated and wild foods from rural areas provide a small share of energy intake, yet they highly contribute micronutrients which are essential for nutrient security. The evidence shows a positive association between TF consumption and micronutrient adequacy. The traditional diet of rural Guasaganda is based on a combination of plant and animal products which need to be balanced towards sustainable diets. However, current fruit and vegetable consumption does not meet the recommendation (each 200 g/d) for the prevention of chronic diseases. Thus, it is suggested to increase the cultivation and consumption of fruits, and particularly vegetables, in home gardens. Furthermore, recommendations include replacing a share of red meat with plant foods to have a traditional diet which is environmentally friendly and helps progress towards Sustainable Development Goal 2.
Acknowledgements
Acknowledgement: The authors are grateful for the advice received from Carl Lachat (Ghent University). Financial support: This work was funded by the International Foundation for Science (grant number E4954-1). The funding covered all fieldwork conducted to collect data, excluding the honoraria of the authors. The International Foundation for Science had no role in the design, analyses or writing of this article. Conflict of interest: No conflicts of interest are perceived. Authorship: The study was designed by D.P., R.E. and P.V.D. Data were collected by D.P. Data analysis was conducted by D.P. (bioscientist) and H.C.-V. (statistician); and R.E. and P.V.D. contributed to the interpretation of results. D.P., H.C.-V., R.E. and P.V.D. wrote the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethical committee of Ghent University (B670201213216) and the Ecuadorian Ministry of Public Health (MSP-DIS0056-2012). Written informed consent was obtained from all subjects.






