Introduction
More than 20% of cancer in the developing world are caused by infections (Brindley et al., Reference Brindley, Costa and Sripa2015). The World Health Organization’s International Agency for Research on Cancer (IARC) recognizes the infection with about 12 pathogens as group 1 biological carcinogens, i.e., definitive causes of cancer. These group 1 agents include three helminth parasites, specifically the fish-borne trematodes (FBT) Opisthorchis viverrini and Clonorchis sinensis and the blood fluke, Schistosoma haematobium (IARC, 2012). In addition, we have previously reported findings from hamster infection that support the inclusion of Opisthorchis felineus, also an FBT, to this list of biological carcinogens and definitive cause of cholangiocarcinoma (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017). We hypothesized that these helminths produce and release derivatives of oestrogens and oxysterols that promote oxidation of host DNA and have the ability of parasite metabolites to directly promote DNA lesions adducts and mutations that ultimately lead to cholangiocarcinoma (Brindley et al., Reference Brindley, Costa and Sripa2015; Costa et al., Reference Costa, Vale, Gouveia, Botelho, Sripa, Santos, Santos, Rinaldi and Brindley2014; Gouveia et al., Reference Gouveia, Santos, Brindley, Rinaldi, Lopes, Santos, da Costa and Vale2015; Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017; Vale et al., Reference Vale, Gouveia, Botelho, Sripa, Suttiprapa, Rinaldi, Gomes, Brindley and Correia da Costa2013). The findings supported the postulate that these infection-associated cancers originate from a biological and/ or chemical insult followed by chronic inflammation, fibrosis, and a change in the tissue microenvironment that leads to a pre-cancerous niche (Brücher & Jamall, Reference Brücher and Jamall2014; Cavalieri et al., Reference Cavalieri, Rogan and Zahid2017). Paradoxically, infections with other close phylogenetic relatives of these carcinogenic helminths, also food borne trematodes of the Phylum Platyhelminthes, have not been categorized as group 1 biological carcinogens (Chapman et al., Reference Chapman1999; Montero et al., Reference Montero, Gentile, Frederick, McMannis, Murphy, Silva, Blankespoor and Gentile1999; Kawanishi et al., Reference Kawanishi, Hiraku, Pinlaor and Ma2006; Kolodziejczyk et al., Reference Kolodziejczyk, Siemieniuk and Skrzydlewska2006; Machicado et al., Reference Machicado, Machicado, Maco, Terashima and Marcos2016; Mayer & Fried, Reference Mayer and Fried2007; Tsocheva-Gaytandzhieva, Reference Tsocheva-Gaytandzhieva2005).
For instance, Fasciola hepatica has a wide geographical range, causes major economic loss in sheep and cattle worldwide, and also is an important zoonosis in humans (Villegas et al., Reference Villegas, Angles, Barrientos, Barrios, Valero, Hamed, Grueninger, Ault, Montresor, Engels, Mas-Coma and Gabrielli2012). It has been shown that fascioliasis can induce host DNA damage through action of reactive nitric species (RNS) or oxygen species (ROS) (Mayer & Fried, Reference Mayer and Fried2007; Tsocheva et al., Reference Tsocheva, Kadiiska, Poljakova-Krusteva, Krustev, Yanev and Stoytchev1992); however, the infection has not been associated to carcinogenesis. Seeking new insights in this apparent paradox among closely related food-borne trematodes, here we conducted an analysis of soluble extracts of adult worms of F. hepatica, O. viverrini and O. felineus by liquid chromatography coupled with mass spectrometry (LC–MS/MS). Remarkably, the LC–MS/MS chromatograms for each of the liver fluke species exhibited clear differences in regard the presence of oxysterols. These metabolites were minor components of the extract from F. hepatica, in contrast to the relatively high abundance and diversity of oxysterols in O. viverrini and O. felineus. The presence of abundant oxysterols in the of Opisthorchis liver flukes would support the hypothesis that these molecules may act as initiators during the liver fluke infection-induced biliary tract malignancy.
Material
Ethics Statement
Procedures undertaken complied with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for animal experiments http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm. Syrian hamsters (Mesocricetus auratus) were purchased from the stock of the Puschino Animal Facility (Russia) and bred at the Animal Facility of the ICG SB RAS (RFMEFI61914X0005) (Russia). The hamsters were maintained according to protocols approved by the Committee on the Ethics of Animal Experiments of the Institute of Cytology and Genetics (Permit Number: 25 of 12.12.2014).
Soluble extracts from F. hepatica, O. viverrini and O. felineus adult liver flukes
Adult worms of F. hepatica were obtained from the bile ducts of infected cattle at a local slaughterhouse (Silva et al., Reference Silva, Castro, Lopes, Rodrigues, Dias, Conceição, Alonso, Correia da Costa, Bastos, Parra, Moradas-Ferreira and Silva2004). It should be noted that the animals were processed as part of normal work of the slaughterhouse. O. viverrini and O. felineus were obtained as previously described (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017; Vale et al., Reference Vale, Gouveia, Botelho, Sripa, Suttiprapa, Rinaldi, Gomes, Brindley and Correia da Costa2013). In brief, metacercariae of Opisthorchis species were obtained from naturally infected cyprinoid fish in Khon Kaen province, Thailand or from naturally infected fish (Leuciscus idus) in the Ob River near the city of Novosibirsk, Siberia Russia, respectively. The fish were digested with pepsin-HCl (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017). Fifty metacercariae were used to infect hamsters (Mesocricetus auratus) and three months after infection, the animals were euthanized and adult O. viverrini or O. felineus flukes recovered from their bile ducts. The worms were washed extensively in phosphate buffered saline (PBS, pH 7.4) supplemented with 100 μg/ml streptomycin and 100 U/ml penicillin G and cultured overnight in serum free RPMI-1640 medium (Lonza, Basel, Switzerland) containing 1% glucose, and protease inhibitors (0.1 mM phenylmethanesulfonyl fluoride, 2 μM E-64 and 10 μM leupeptin) (Sigma-Aldrich, St. Louis, Missouri) at 37 °C, 5% CO2.
Soluble extracts from all samples were prepared by sonication (5 x 5 s burst, output cycle 4, Branson Sonifier 450, Germany) in PBS supplemented with protease inhibitors [500 μM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), 0.3 μM aprotinin, 10 μM E-64, 10 μM bestatin and 10 μM leupeptin] (M221, Amresco, Solon, OH, USA), followed by 30 min centrifugation at 10,000 rpm, 4 °C. The protein concentration of supernatants was determined using a commercial kit. Ascorbic acid was added to 1 mg/ml to these extracts, which were stored in aliquots at −80 °C (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017; Vale et al., Reference Vale, Gouveia, Botelho, Sripa, Suttiprapa, Rinaldi, Gomes, Brindley and Correia da Costa2013).
Sample preparation and LC–MS/MS analysis
Samples were prepared and processed using liquid chromatography diode array detection electron spray ionization mass spectrometry, as previously described (Gouveia et al., Reference Gouveia, Santos, Brindley, Rinaldi, Lopes, Santos, da Costa and Vale2015; Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017; Vale et al., Reference Vale, Gouveia, Botelho, Sripa, Suttiprapa, Rinaldi, Gomes, Brindley and Correia da Costa2013). Due to the acceptable chromatographic performance of methanol as the solvent in terms of separation and sensitivity, with short gradient times (Wang et al., Reference Wang, Shibue and Gross2000), this solvent was added up to 20% (v/v). High performance liquid chromatography coupled with mass spectrometer was employed to investigate molecular species from liver flukes, with samples of 25 μl injected into the LC–MS/MS instrument for analysis. The mass analysis was performed within an LTQ Orbitrap XL mass spectrometer (Thermo Fischer Scientific, Bremen, Germany), fitted with an ultraviolet (UV) photo diode array (PDA) detector. Analysis involved a Macherey-Nagel Nucleosil C18-column (250 mm x 4 mm internal diameter; 5 μm particle diameter, end-capped), proceeding at a flow rate of 0.3 ml/min. The capillary voltage of the electrospray ionization was 28 kW, capillary temperature was 310 °C, flow rates of the sheath gas and auxiliary N2 were set to 40 and 10 (arbitrary unit as provided by the software settings), respectively, and gas temperature was 275 °C (Gouveia et al., Reference Gouveia, Santos, Brindley, Rinaldi, Lopes, Santos, da Costa and Vale2015; Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017; Vale et al., Reference Vale, Gouveia, Botelho, Sripa, Suttiprapa, Rinaldi, Gomes, Brindley and Correia da Costa2013). The mobile phase consisted of 1% formic acid in water (A)/acetonitrile (B) mixtures. Eluates were monitored for 75 min, run with a mobile phase gradient of 0–5 min, 100% A; 5–10 min, linear gradient from 100% to 80% A, 10–15 min 80% A, 15–50 min, linear gradient from 80% to 40% A; 50–65 min, 40% A; 65–75 min, linear gradient from 40% to 100% B. Washing for 15 min with acetonitrile was carried out to stabilize the column. Data were collected in negative electrospray ionization negative mode scanning a mass to charge ratio (m/z) range of 50–2,000.
Results
Both species of Opisthorchis shared identical mass spectra profiles
We have developed a sensitive LC–MS/MS-based protocol to identify new steroids-derived molecules not only in extracts of helminth parasites (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017; Vale et al., Reference Vale, Gouveia, Botelho, Sripa, Suttiprapa, Rinaldi, Gomes, Brindley and Correia da Costa2013), but also from experimental infected rodents (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017) and naturally-infected humans (Gouveia et al., Reference Gouveia, Santos, Brindley, Rinaldi, Lopes, Santos, da Costa and Vale2015). Extracts obtained from F. hepatica adult worms were analyzed in order to provide insights related to their composition and complexity.
Comparing data obtained for O. viverrini with O. felineus we observed that both these liver flukes displayed highly similar mass spectra (MS) and shared most peaks detected (indicated in grey in Fig 1) which were attributed to oxysterol-like metabolites, e.g. mass/charge (m/z) 356, 307, bile acids in oxidized form, e.g. m/z 443, 479, 488 and DNA-adducts, e.g. m/z 599, 639, 667 (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017; Vale et al., Reference Vale, Gouveia, Botelho, Sripa, Suttiprapa, Rinaldi, Gomes, Brindley and Correia da Costa2013).

Fig. 1 Comparison of mass spectral profiles obtained for Fasciola hepatica and Opisthorchis spp. Panel A, common m/z between the three liver flukes; panel B, major differences among the liver flukes.
F. hepatica extracts exhibited striking differences to those of the Opisthorchis species
Notable differences were apparent among the MS profiles of F. hepatica and the Opisthorchis species. Most of compounds present in both Opisthorchis species were absent from F. hepatica, specifically m/z 356, 357, 425 and 307. Remarkably, these specific compounds were attributed to be oxysterols with ability to react with host DNA as described (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017). The MS profile of F. hepatica was much more complex than those obtained for Opisthorchis spp. (Fig. 1). The major differences were observed at retention intervals of approximately 24, 32, and 40 min – as indicated in orange, yellow and blue, respectively, on the chromatographs (Fig. 1). On these retention times, F. hepatica showed greater number of compounds in comparison to those observed on Opisthorchis species (Fig. 1 and Table 1). Remarkably, most of these compounds were detected only in F. hepatica extracts (Table 1).
Table 1. Comparison of mass/charge (m/z) obtained for Fasciola hepatica during this study with Opisthorchis spp. previously reported for O. viverrini (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017; Vale et al., Reference Vale, Gouveia, Botelho, Sripa, Suttiprapa, Rinaldi, Gomes, Brindley and Correia da Costa2013) and O. felineus (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017). The structures of common m/z (signed at green) are depicted on Table 2.

Table 2. Structures of m/z common to Fasciola hepatica and Opisthorchis species.

Unlike Opisthorchis, F. hepatica displayed a higher number of compounds with elevated m/z (between 600 and 800), mostly between retention interval of 38 to 42 min (Table 1), which might suggest that they are more complex than the majority of those detected on Opisthorchis spp.
Nonetheless, F. hepatica and Opisthorchis spp. shared several common compounds at retention interval of 58–64 min (signed by green in Fig. 1 and Table 1). These compounds have been ascribed previously to oxysterol-like metabolite (e.g. m/z 325), bile acids (e.g. m/z 571) and as well as DNA-adducts (m/z 599) (Vale et al., Reference Vale, Gouveia, Botelho, Sripa, Suttiprapa, Rinaldi, Gomes, Brindley and Correia da Costa2013) which is expected since parasites reside on bile ducts. Remarkably, however, oxysterol-like metabolites, bile acids and DNA-adducts in particular were fewer in F. hepatica compared to Opisthorchis spp. The fact, F. hepatica has lesser oxysterol-like metabolites might be one of the reasons that explain why a carcinogenic potential associated with its infection has not been established.
Discussion
Both chronic infection with Fasciola spp. and Opisthorchis spp. could lead to fibrosis, hyperplasia, and biliary stasis (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017; Machicado et al., Reference Machicado, Machicado, Maco, Terashima and Marcos2016; Maksimova et al., Reference Maksimova, Pakharukova, Kashina, Zhukova, Kovner, Lvova, Katokhin, Tolstikova, Sripa and Mordvinov2017; Motorna et al., Reference Motorna, Martin, Gentile and Gentile2001; Sithithaworn et al., Reference Sithithaworn, Andrews, Nguyen, Wongsaroj, Sinuon, Odermatt, Nawa, Liang, Brindley and Sripa2012). However, an association between fascioliasis and cancer remains controversial and not definitely established (Machicado et al., Reference Machicado, Machicado, Maco, Terashima and Marcos2016). Thus, we decided to investigate extracts of adult worms of F. hepatica and compare with data previously obtained for Opisthorchis spp. We aimed to address the following questions: 1) does F. hepatica synthesize and excrete metabolites that might promote direct damage on host DNA? The MS profile of F. hepatica was found to be far more complex, showing an elevated number of compounds more complex that translate in an elevated m/z rather than Opisthorchis spp. This suggested that metabolic process that occur in F. hepatica are dissimilar to those in Opisthorchis spp. and also could be related to its complex route of migration to the biliary tract might underlie these differences.
Compounds of F. hepatica might be related to the different migratory route of the parasite to the biliary tree. Unlike Opisthorchis spp., newly excysted juveniles of F. hepatica exit the lumen of the small intestine, transverse the intestinal wall and migrate through the abdominal cavity to the Glisson’s capsule of the liver (Mas-Coma, Reference Mas-Coma2005; Moazeni & Ahmadi, Reference Moazeni and Ahmadi2016). This parasite might deploy more complex biochemical processes and secretions, including the secretion of cathepsins (Cancela et al., Reference Cancela, Ruétalo, Dell’Oca, da Silva, Smircich, Rinaldi, Roche, Carmona, Alvarez-Valín, Zaha and Tort2010; Cwiklinski et al., Reference Cwiklinski, Dalton, Dufresne, La Course, Williams, Hodgkinson and Paterson2015; Young et al., Reference Young, Hall, Jex, Cantacessi and Gasser2010) to accomplish this elaborate organ and tissue migration. The juvenile F. hepatica infects the liver by directly penetrating the Glisson’s capsule from the abdominal cavity, and thereafter burrows through the hepatic parenchyma to the bile ducts where it eventually matures into the egg-laying adult worm (Moazeni & Ahmadi, Reference Moazeni and Ahmadi2016). Components detected in the extracts of F. hepatica might be related with digestion of host tissues including blood such as hemoglobin, albumin and immunoglobin to support reproductive process including synthesis of eggs (Moazeni & Ahmadi, Reference Moazeni and Ahmadi2016). On other hand, most of the compounds observed from 23 to 57 minutes were specific of F. hepatica, i.e. not present in Opisthorchis. Juvenile Opisthorchis flukes ascend from the duodenum directly into the lumen of biliary tree (Sithithaworn et al., Reference Sithithaworn, Andrews, Nguyen, Wongsaroj, Sinuon, Odermatt, Nawa, Liang, Brindley and Sripa2012; Pakharukova et al., Reference Pakharukova and Mordvinov2016).
Glycocholic acid in the mammalian small intestine triggers the excystment of the metacercaria and emergence of F. hepatica juvenile flukes stimulating the exit of the parasite from the gut lumen and its migration to the abdominal cavity. Intriguingly, the juvenile F. hepatica did not survive in bile-containing solutions whereas the adult fluke resides in the bile ducts, bathed in bile (Tielens et al., Reference Tielens, Van der Meer and Van den Bergh1981). Differences in the nature of the juvenile versus adult tegument of F. hepatica and the selectivity and the permeability of glycocalyx of the tegument may underpin these stage specific differences (Tielens et al., Reference Tielens, Van der Meer and Van den Bergh1981). The complexity of the tegument, a complex metabolically active and highly glycosylated biological matrix (Ravidá et al., Reference Ravidá, Cwiklinski, Aldridge, Clarke, Thompson, Gerlach, Kilcoyne, Hokke, Dalton and O’Neill2016) might also underpin complexity of F. hepatica MS profile and its components.
Some of these compounds might be precursors of known compounds that were previously attributed to oxysterol-like metabolites, bile acids and DNA-adducts (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017; Vale et al., Reference Vale, Gouveia, Botelho, Sripa, Suttiprapa, Rinaldi, Gomes, Brindley and Correia da Costa2013). This is feasible since all three flukes live within the biliary tree. There is evidence that F. hepatica induces DNA damage through the action of mutational mediators (Jedina et al., Reference Jedina, Kozak-Ljunggren and Wedrychowicz2011; Kolodziejczyk et al., Reference Kolodziejczyk, Siemieniuk and Skrzydlewska2006). The presence of DNA adducts in tissue does not necessarily imply a specific tumorigenic risk for the host tissue. Other factors such as DNA repair and cell proliferation key roles players in determining the overall carcinogenic risk (Povey, Reference Povey2000). An association between fascioliasis and cancer has only been suggested from in vitro studies and, thus far, there have not been satisfactory reports of human cases of bile duct cancer due to chronic infection with F. hepatica (Chun et al., Reference Chun, Bae, Yun, Yang and Kong2012; Gentile et al., Reference Gentile, Gentile, Nannenga, Johnson, Blankespoor and Montero1998; Hanahan & Weinberg, Reference Hanahan and Weinberg2011; Machicado et al., Reference Machicado, Machicado, Maco, Terashima and Marcos2016; Tsocheva-Gaytandzhieva, Reference Tsocheva-Gaytandzhieva2005). Therefore, there is a lack of cogent evidence that relate fascioliasis with cancer (Machicado et al., Reference Machicado, Machicado, Maco, Terashima and Marcos2016). By contrast, a number of reports posit opposing effects, i.e. tumor growth stimulation and inhibition. Tumor growth stimulation and proliferation of hepatocytes has been observed during acute phase of infection where larval flukes migrate through the parenchyma of the liver and provoke marked inflammation (Montero et al., Reference Montero, Gentile, Frederick, McMannis, Murphy, Silva, Blankespoor and Gentile1999; Tsocheva et al., Reference Tsocheva, Kadiiska, Poljakova-Krusteva, Krustev, Yanev and Stoytchev1992). In turn, the chronic inflammation increases oxidative stress that can overwhelm antioxidant system homeostasis to dampen reactive oxygen species and consequent oxidative modification of lipids, nucleic acids and proteins (Kolodziejczyk et al., Reference Kolodziejczyk, Siemieniuk and Skrzydlewska2006). Like fascioliasis, opisthorchiasis is characterized by elevated oxidative stress and altered the antioxidant systems (Kawanishi et al., Reference Kawanishi, Hiraku, Pinlaor and Ma2006; Kolodziejczyk et al., Reference Kolodziejczyk, Siemieniuk and Skrzydlewska2006). We also documented that infection with O. felineus induces biliary intraepithelial neoplasia (BilIN). The consonance of findings that the presence of new metabolites and of BilIN-1 and BilIN-2 indicates that O. felineus infection induces neoplastic transformation of cholangiocytes and can be expected to promote growth of biliary cancers (Gouveia et al., Reference Gouveia, Pakharukova, Laha, Sripa, Maksimova, Rinaldi, Mordvinov, Amaro, Santos, Costa and Vale2017). Tumor inhibition has been noted during the chronic phase of fascioliasis that may dampen the liver metabolizing activity (Montero et al., Reference Montero, Gentile, Frederick, McMannis, Murphy, Silva, Blankespoor and Gentile1999). Whereas acute F. hepatica infection may increase the metabolizing enzymes in liver and thus increase the activation of exogenous carcinogens (Motorno et al., Reference Motorna, Martin, Gentile and Gentile2001), chronic infection may reduce hepatic metabolizing activity (Montero et al., Reference Montero, Gentile, Frederick, McMannis, Murphy, Silva, Blankespoor and Gentile1999). It is noteworthy that chronic infection with F. hepatica in a rat model suppressed N-nitrosodimethyldiamine-induced carcinogenesis, suggesting a parasite-induced inhibition of carcinogenesis in the liver of rodents experimentally infected with F. hepatica (Tsocheva et al., Reference Tsocheva, Kadiiska, Poljakova-Krusteva, Krustev, Yanev and Stoytchev1992). Could this ability be one of the reasons why the infection with F. hepatica does not present carcinogenic potential? Is F. hepatica able to neutralize the reactivity of oxysterol-like molecules or other carcinogenic with host DNA? All these questions require further investigation. This study aimed to characterize the differences between Fasciola and Opisthorchis with a view to identifying the parasite-derived compound that results in the different pathogenic outcomes after infection with these two parasites. Not only is this important to understand mechanisms underlying the pathology, with a view to perhaps developing appropriate therapeutics in the future, but it would also provide information to understand how two flukes have adapted to induce such different outcomes in their hosts. Future studies will evaluate if there is a host-related effect on the production of the metabolites identified in this study.
In conclusion, we discovered that Fasciola hepatica displayed more complex mass spectra profile that the Opisthorchis species and several specific compounds that might be related to its complex route of migration to the biliary tract. Nonetheless, F. hepatica shared few compounds with Opisthorchis species, which are related to oxysterols, bile acids and DNA-adducts. The presence of only a few common compounds might explain why fascioliasis has not been causally linked with liver cancer. Indeed, we posit that presence of fewer oxysterol-like metabolites might (partially) explain why definitive carcinogenic potential has not been ascribed to ruminant or human fascioliasis (Fig. 2). More study can be expected to enhance our understanding of the differences in carcinogenicity between these two genera of food borne liver flukes.
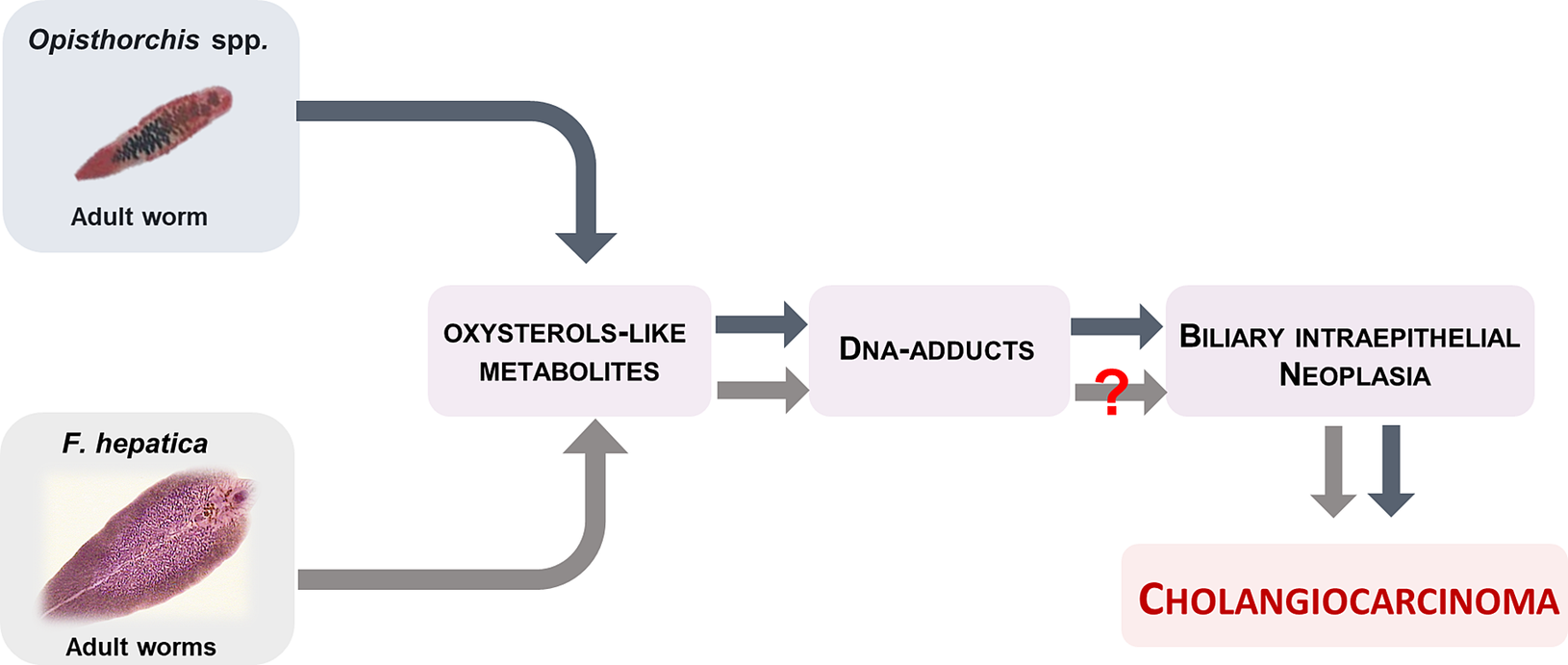
Fig. 2 Adult liver flukes O. viverrini and O. felineus produces oxysterol-like metabolites that interact with host chromosomal DNA to form DNA-adducts and forms of biliary intraepithelial neoplasia that conducive to cholangiocarcinoma. F. hepatica also elaborates oxysterol-like metabolites, but at much lower number, which might be explain, at least in part, why infection with this parasite fails to induce malignancy.
Acknowledgments
NV thanks Fundação para a Ciência e a tecnologia (FCT, Portugal) and Fundação Manuel António da Mota (FMAM, Portugal) by support Nuno Vale Lab. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the FCT or FMAM.
Author Contributions
M.J.G. and N.V. conceived and planned the experiments. M.J.G., M.P. and N.V. carried out the experiments. M.J.G. contributed to sample preparation. M.J.G., M.P., G.R., P.B. and N.V. contributed to the interpretation of the results. M.J.G. and N.V. took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Funding Information
This work was financed by FEDER - Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 - Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT - Fundação para a Ciência e a Tecnologia, in the framework of the project, Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274). The FCT and FEDER (European Union) also supported these studies through project number IF/00092/2014/CP1255/CT0004. This work was also supported in part by the Russian Science Foundation [grant number 18–15-00098 (VAM)].
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.







Comments
Comments to the Author: This works is designed to compare the spectrometric profiles of soluble extracts from F. hepatica, O. viverrini and O. felineus. The authors found 3 common compounds: oxysterol-like metabolites, bile acids and DNA-adducts.
They believe the first compound related to carcinogenesis. The findings are interesting and could explain the different/ common pathogenesis caused by these flukes. However some points should be revised or clarified.
- Fig. 2 : Because the carcinogenesis is multistage-multistep process, other important factors should be added in the diagram.
- Does the host spp. affect the results? F. hepatica adult worms are from natural host but O. viverrini and O. felineus worms are from animal model.