Resveratrol (3,5,4'-trihydroxystilbene) is the most well-known stilbene. This polyphenol class also includes piceid (resveratrol glucoside), pallidol and viniferins (resveratrol oligomers), and piceatannol (tetrahydroxystilbene). In the last two decades, resveratrol and other stilbenes have received considerable attention, particularly in in vitro and preclinical studies, because of their various biological properties such as antioxidant and anti-inflammatory effects( Reference Baur and Sinclair 1 , Reference Tome-Carneiro, Larrosa and Gonzalez-Sarrias 2 ). Their capacity to modulate several signalling pathways leads to important cellular processes such as apoptosis, inhibition of angiogenesis and tumour growth as well as lipidic and glycaemic modulation( Reference Baur and Sinclair 1 , Reference Tome-Carneiro, Larrosa and Gonzalez-Sarrias 2 ). However, evidence from epidemiological studies regarding effects on health is lacking, because of the difficulty in accurately estimating resveratrol exposure in large population-based samples, and because of the low levels of resveratrol observed in humans in non-experimental conditions compared with those in experimental conditions( Reference Tome-Carneiro, Larrosa and Gonzalez-Sarrias 2 , Reference Rabassa, Zamora-Ros and Urpi-Sarda 3 ).
Resveratrol and its derivatives are found in at least seventy-two plant species, including twelve families and thirty-one genera, but they are found in only a limited number of edible foods (online Supplementary Table S1)( Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 4 , Reference Neveu, Perez-Jimenez and Vos 5 ). The primary sources of stilbenes are grapes and wine, especially red wine( Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 4 , Reference Neveu, Perez-Jimenez and Vos 5 ). They can also be found in minor concentrations in peanuts, pistachios, berries( Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 4 , Reference Neveu, Perez-Jimenez and Vos 5 ), dark chocolate( Reference Hurst, Glinski and Miller 6 ), some beers( Reference Chiva-Blanch, Urpi-Sarda and Rotches-Ribalta 7 ) and in some varieties of tomatoes( Reference Ragab, Van and Jankowski 8 ) and apples( Reference Farneti, Masuero and Costa 9 ). Dietary intake of stilbenes in Europe is between 2 and 3 mg/d, and resveratrol and piceid represent about 50 % of the total stilbene intake( Reference Zamora-Ros, Knaze and Rothwell 10 ).
Resveratrol and piceid, after hydrolysis, are absorbed in the small intestine and extensively metabolised into glucuronides and sulphates. Unabsorbed resveratrol reaches the colon and is converted into dihydroresveratrol by the microbiota, which can also be absorbed, accounting for as much as 50 % of an oral resveratrol dose( Reference Walle, Hsieh and DeLegge 11 ). Experiments with radiolabelled resveratrol have shown a high absorption of resveratrol in the gut, with more than 70 % of the ingested radioactivity recovered in urine( Reference Walle, Hsieh and DeLegge 11 ). To date, very little information is available on the bioavailability of other stilbenes such as piceatannol( Reference Lin, Tringali and Spatafora 12 ) and viniferins( Reference Willenberg, Michael and Wonik 13 ); although, to the best of our knowledge, they are not parent compounds of urinary resveratrol( Reference Rothwell, Urpi-Sarda and Boto-Ordonez 14 ).
Few studies assessing the ability of urinary resveratrol to serve as a biomarker of wine and resveratrol intake have been published. These studies have been conducted in Mediterranean countries where wine intake is high( Reference Sieri, Agudo and Kesse 15 ). One interventional( Reference Zamora-Ros, Urpi-Sarda and Lamuela-Raventos 16 ) and two observational studies( Reference Zamora-Ros, Urpi-Sarda and Lamuela-Raventos 17 , Reference Semba, Ferrucci and Bartali 18 ) showed high correlations between habitual intake of wine or resveratrol and piceid and the concentration of resveratrol metabolites in spot urine samples. High correlations were also observed between dihydroresveratrol glucuronide and resveratrol concentrations measured in 24-h urine samples and acute intake of wine on the day of urine collection in a cross-sectional study in four European countries( Reference Edmands, Ferrari and Rothwell 19 , Reference Zamora-Ros, Achaintre and Rothwell 20 ).
In the present study, we aimed to investigate the correlation between acute and habitual dietary intakes of resveratrol and stilbenes, and their main food sources, and concentrations of urinary resveratrol in 24-h urine. First, our study assessed the usefulness of urinary resveratrol as a biomarker of resveratrol and wine consumption. Second, it evaluated the validity of using a dietary questionnaire (DQ) and the Phenol-Explorer food composition database( Reference Neveu, Perez-Jimenez and Vos 5 ) to estimate the dietary resveratrol intake.
Methods
Study population
The European Prospective Investigation into Cancer and Nutrition (EPIC) study is a cohort study with over half a million participants of both sexes mostly recruited from the general population between 1992 and 2000 in twenty-three centres in ten European countries( Reference Riboli and Kaaks 21 ). In a convenience subsample of 475 men and women, aged 50–61 years, a single 24-h dietary recall (24-HDR) and 24-h urine samples were collected on the same day between the years 1995 and 1999( Reference Zamora-Ros, Achaintre and Rothwell 20 ). Approval for the study was obtained from the ethical review boards of the International Agency for Research on Cancer and from all participating institutions. All participants also provided written informed consent.
Dietary and lifestyle information
Short-term dietary data were collected using a single 24-HDR harmonised across countries (EPIC-Soft)( Reference Slimani, Ferrari and Ocke 22 ). The 24-HDR was administered in a face-to-face interview. Habitual dietary data were assessed at recruitment using a centre-specific quantitative DQ (France, Germany, Greece and Italy, except Naples) or a semi-quantitative FFQ (Naples) developed and validated in each country/centre( Reference Riboli and Kaaks 21 , Reference Margetts and Pietinen 23 ). The mean time interval between the DQ and the 24-HDR interview varied by country, from 1 d to 3 years later( Reference Slimani, Kaaks and Ferrari 24 ). Dietary resveratrol and resveratrol 3-O-glucoside (piceid) intakes were estimated using Phenol-Explorer( Reference Neveu, Perez-Jimenez and Vos 5 ), a comprehensive food composition database on polyphenols, as described in detail elsewhere( Reference Zamora-Ros, Knaze and Rothwell 10 ). Resveratrol aglycone equivalents were calculated as the sum of resveratrol and piceid contents after conversion of piceid to resveratrol equivalents on the basis of molecular weight. Total energy intake was estimated using the standardised EPIC Nutrient Database( Reference Slimani, Deharveng and Unwin 25 ).
Data on education, physical activity and smoking history were collected at baseline using questionnaires( Reference Slimani, Kaaks and Ferrari 24 , Reference Wareham, Jakes and Rennie 26 ). Data on age, body weight and height were self-reported by study participants during the 24-HDR interview.
Samples and analytical method
Urine samples over a 24-h period were collected and stored at −20°C using boric acid as a preservative. Completeness of the collection of 24-h urine samples was monitored using p-aminobenzoic acid (PABA). Samples with PABA recovery <70 and >110 % were excluded from this study( Reference Slimani, Bingham and Runswick 27 ).
Resveratrol was analysed in 24-h urine samples using ultra-performance liquid chromatography–tandem MS( Reference Achaintre, Bulete and Cren-Olive 28 ). In brief, urine samples were treated with a β-glucuronidase/sulfatase enzyme mixture and extracted twice with ethyl acetate; all phenolic groups in resveratrol were quantitatively dansylated using a differential isotope-labelling method and quantified using tandem MS. The limit of quantification (LOQ) for resveratrol was 0·02 μm. Inter-assay and intra-assay CV were <10 %. Urinary excretion of resveratrol was expressed as μmol/24-h. A small proportion of urinary excretion measurements (n 46) did not meet predetermined analytical quality control criteria and were therefore excluded from statistical analysis.
Statistical analyses
Urinary resveratrol concentrations that fell below the LOQ were set to values corresponding to half the LOQ. Descriptive statistics (medians and 10th and 90th percentiles) including consumers and non-consumers were used for both urinary excretions and dietary intakes. Kruskal–Wallis tests were used to compare urinary resveratrol levels between groups of subjects with different baseline demographics and lifestyle characteristics. Spearman’s rank correlations were used to assess the association between urinary resveratrol levels and dietary variables (stilbenes and stilbene-rich foods) estimated using DQ and 24-HDR. Partial Spearman’s correlations (R 2 partial) were computed to assess the contribution of dietary resveratrol intake (using DQ or 24-HDR) to urinary resveratrol levels while adjusting for other possible confounders: sex, BMI, age at recruitment, smoking status (never smoker, former smoker, current smoker) and total energy intake (measured in the DQ or in the 24-HDR, as appropriate). All statistical tests were two sided and the significance level was P<0·05. All analyses were conducted using the Statistical Analysis Software, release 9.3 (SAS Institute Inc.).
Results
Of the 429 subjects with urinary resveratrol data, fifty-two participants had urinary resveratrol concentrations <LOQ. The median urinary excretions by centre, sex, age, BMI, smoking status and total energy intake are shown in Table 1. Excretion was higher in male subjects, in those aged 50–60 years and in those who were overweight (BMI 25–<30 kg/m2) or had greater energy intake. The highest total urinary resveratrol concentrations were observed in Italy and France (0·09–0·19 μmol/24 h), whereas the lowest concentrations were observed in Greece (0·04 μmol/24 h). A similar pattern of habitual wine consumption by country was observed; the median intake ranged from 13 ml/d in Greece to 125 ml/d in Italian centres (data not tabulated).
Table 1 Urinary resveratrol excretion (μmol/24 h) according to sociodemographic and lifestyle factors in the European Prospective Investigation into Cancer and Nutrition study (Medians and 10th and 90th percentiles (P10 and P90))

FRA, France; ITA, Italy; GER, Germany.
Medians and 10th and 90th percentiles of the intake of resveratrol and resveratrol 3-O-glucoside and their food sources (e.g. wine, grapes, peanuts and red wine) are described in Table 2. Medians of the sum of resveratrol and piceid intake obtained using the 24-HDR were lower (0·10 mg/d) than those obtained using the DQ (0·42 mg/d). As expected, a higher number of subjects with negligible intake of the sum of resveratrol and piceid was found using the 24-HDR (n 82) than using the DQ (n 0). Similar results were observed for wine, with medians varying between 0 g/d (276 non-wine consumers) and 45 g/d (seventy non-wine consumers) in the 24-HDR and in the DQ, respectively.
Table 2 Dietary intakes of resveratrol and piceid, their food sources and urinary excretion of resveratrol in the European Prospective Investigation into Cancer and Nutrition study (n 475) (Medians and 10th and 90th percentiles (P10 and P90))

LOQ, limit of quantification; DQ, dietary questionnaire.
* Peanut data only collected using the DQ in Germany (n 177).
† Red wine data only collected using the DQ in Italy, except Naples (n 155).
Spearman’s correlation coefficients between urinary excretion and intakes of different dietary stilbenes were >0·61 and >0·55 as reported using 24-HDR and DQ (Table 3), respectively. The correlation between the sum of resveratrol and piceid intake and wine consumption was found to be 0·83 using 24-HDR and 0·95 using DQ. DQ data on red wine were collected only in Italy (except in Naples); therefore, data were available for 155 participants only. Correlations between red wine consumption and the sum of resveratrol and piceid intake were found to be lower in the 24-HDR (r 0·71) than in the DQ (r 0·96). No significant correlations were found with either grape or peanuts. DQ data on peanut intake were collected only in Germany (n 177). The consumption of peanuts was negligible (0 g/d in the percentile 90 using both DQ and 24-HDR), and the number of peanut consumers was only fourteen of 475 subjects using the 24-HDR and thirty-three of 177 individuals using the DQ (Table 2).
Table 3 Spearman’s correlations between urinary resveratrol (RESV) concentrations and acute intakes of RESV, RESV 3-O-glucoside and their main food sources estimated with a single 24-h dietary recall and a dietary questionnaire in the European Prospective Investigation into Cancer and Nutrition study
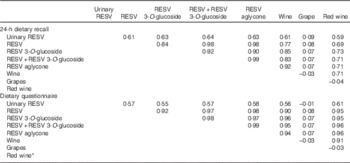
* Red wine data only collected using the dietary questionnaire in Italy, except Naples (n 155).
The results of the multivariate-adjusted partial Spearman’s correlations (R 2 partial) between the intake of resveratrol and piceid, and their main food sources, and urinary resveratrol are shown in Table 4. R 2 partial coefficients were similar (R 2 partial approximately 0·6) with resveratrol, piceid and wine using the 24-HDR. R 2 partial coefficients were slightly lower (R 2 partial approximately 0·5) using data from the DQ. For red wine, the R 2 partial coefficients were 0·55 and 0·50 for 24-HDR and DQ, respectively.
Table 4 R 2 partial coefficients between dietary resveratrol and resveratrol 3-O-glucoside intake and urinary resveratrol concentrations in the European Prospective Investigation into Cancer and Nutrition study (n 429)Footnote *
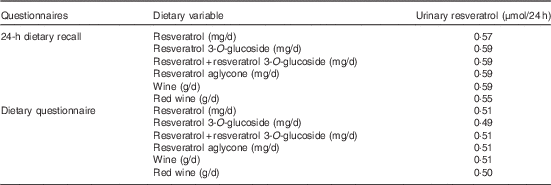
* Spearman’s correlation model adjusted for centre, sex, age, BMI, smoking status and total energy intake.
Discussion
In the current study, resveratrol concentrations in 24-h urine were correlated with acute (R 2 partial approximately 0·6) and habitual (R 2 partial approximately 0·5) dietary resveratrol, piceid and wine intake in a multicentre cohort of European adults. Habitual intake of the sum of resveratrol and piceid has previously been reported to correlate with concentrations of resveratrol metabolites (r 0·67), glucuronides and sulphates analysed in 24-h urine samples in a subsample of 783 men and women, aged 65 years and older, in the Italian InCHIANTI (Invecchiare in Chianti) study( Reference Semba, Ferrucci and Bartali 18 ). Higher correlations (r 0·89) were observed between concentrations of resveratrol metabolites in morning urine samples and the habitual intake of resveratrol and piceid in a subsample of 1000 high-cardiovascular-risk men and women in the Spanish PREDIMED (Prevención con Dieta Mediterránea) study( Reference Zamora-Ros, Urpi-Sarda and Lamuela-Raventos 17 ). The high correlation in the Spanish study could be due to the large proportion of red wine consumers (77 %), whereas in our study the percentage of red wine consumers was much lower (37 %). In agreement with this hypothesis, we observed higher Spearman’s correlations in countries with high wine consumption, such as Italy (r 0·62, 38 % non-wine consumers), than in Germany (r 0·38, 78 % non-wine consumers). Therefore, urinary resveratrol concentration is a more suitable biomarker of resveratrol exposure in populations with elevated and regular intakes of resveratrol/wine than in those with low and occasional intakes.
Approximately 70 % of dietary resveratrol and piceid consumed is recovered in urine as conjugates of resveratrol and dihydroresveratrol produced by the microbiota, which account for as much as 50 % of an oral resveratrol dose( Reference Walle, Hsieh and DeLegge 11 ). Because of extensive metabolism in the gut and the liver, most of the resveratrol found in urine is glucuronidated and/or sulphated( Reference Rotches-Ribalta, Andres-Lacueva and Estruch 29 ), and only 1 % of resveratrol absorbed is found in its free form in urine( Reference Walle, Hsieh and DeLegge 11 ). Therefore, in the analysis of urinary resveratrol, it is common practice to measure it after enzymatic hydrolysis to release resveratrol aglycone, as done here( Reference Achaintre, Bulete and Cren-Olive 28 ). The current analytical method allowed us to accurately quantify urinary resveratrol using a proper standard, whereas, to date, standards for all conjugated metabolites of resveratrol are not commercially available. In addition, this method simultaneously analyses thirty-four urinary polyphenol aglycones, allowing us to potentially measure several nutritional biomarkers of polyphenol-rich foods at the same time( Reference Zamora-Ros, Achaintre and Rothwell 20 ).
Assessment of resveratrol intake in populations represents a challenge for researchers primarily because of the variability and paucity of data on resveratrol content of some foods, although wine, particularly red wine, has been shown to be the main food source (>92 %) for total resveratrol and piceid intakes( Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 4 , Reference Zamora-Ros, Knaze and Rothwell 10 ). Resveratrol has been identified only in very few foods, mainly grapes, grape juice and wine. Chocolate, pistachios, peanuts and some varieties of berries( Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 4 , Reference Neveu, Perez-Jimenez and Vos 5 ), beer( Reference Chiva-Blanch, Urpi-Sarda and Rotches-Ribalta 7 ), tomatoes( Reference Ragab, Van and Jankowski 8 ) and apples( Reference Farneti, Masuero and Costa 9 ) also contain resveratrol, but in very small concentrations, or they are rarely consumed (i.e. berries) (online Supplementary Table S1). Moreover, resveratrol content can vary greatly in the same type of fruit, depending on variety, degree of maturity at harvest, fungal pressure and climate( Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 4 , Reference Neveu, Perez-Jimenez and Vos 5 ). Indeed, resveratrol content in red wines may vary up to 10-fold mainly owing to grape variety, Pinot noir being the variety with the highest content( Reference Lamuela-Raventós, Romero-Pérez and Waterhouse 30 ). The development of Phenol-Explorer in 2009 has facilitated the assessment of resveratrol intake in epidemiological studies( Reference Neveu, Perez-Jimenez and Vos 5 ). However, to our knowledge, this is the first study correlating the estimation of dietary resveratrol intake using Phenol-Explorer with urinary resveratrol concentrations.
In the current study, similar correlations were observed between urinary resveratrol concentrations and dietary intake of either the sum of or individual resveratrol and piceid, or of resveratrol and piceid expressed as resveratrol aglycone equivalents. Therefore, urinary resveratrol excretion is a good predictor of dietary resveratrol and piceid. This is because wine, particularly red wine, is the main food source of resveratrol and piceid (>92 %)( Reference Zamora-Ros, Andres-Lacueva and Lamuela-Raventos 4 , Reference Zamora-Ros, Knaze and Rothwell 10 ). Likewise, in our study, urinary resveratrol level was also significantly correlated with wine consumption, especially with red wine intake, as previously described in other studies( Reference Zamora-Ros, Urpi-Sarda and Lamuela-Raventos 16 , Reference Zamora-Ros, Urpi-Sarda and Lamuela-Raventos 17 ). Other non-resveratrol-related metabolites such as gallic acid ethyl ester, gallic acid ethyl ester sulphate, methylgallic acid sulphate, 4-O-methylgallic acid and hydroxytyrosol sulphate were also suggested as adequate biomarkers of red wine( Reference Edmands, Ferrari and Rothwell 19 , Reference Slimani, Ferrari and Ocke 22 ). Combinations of several metabolites could be better biomarkers of wine intake than individual compounds( Reference Noh, Freisling and Assi 31 ).
Urinary excretion mainly takes place in the first 4 h after consumption (77 % of total excretion), although resveratrol metabolites remain in urine for at least 24 h after intake( Reference Rotches-Ribalta, Andres-Lacueva and Estruch 29 , Reference Boocock, Faust and Patel 32 ). Therefore, correlations between urinary and dietary resveratrol were found to be higher when using 24-HDR (R 2 partial approximately 0·6), a dietary assessment tool for short-term and acute dietary intake, than when using DQ (R 2 partial approximately 0·5), a tool for long-term and habitual dietary intake. Similar findings with other polyphenols comparing short- with long-term dietary exposures have been reviewed previously( Reference Zamora-Ros, Touillaud and Rothwell 33 ). Despite this, partial correlations using DQ were still significant. This is due to the fact that wine was consumed on a regular basis by European adults in the 1990s, especially in wine-producing countries such as France, Italy and Spain. For example, the number of red wine consumers was 121 of 475 and 128 of 155 subjects (data collected only in some Italian centres) on using the 24-HDR and DQ, respectively. Moreover, using urinary resveratrol metabolites as biomarkers of wine consumption, Zamora-Ros et al. were able to differentiate non-wine consumers from sporadic consumers (one to three glasses of wine per week) who had consumed their last glasses 3–5 d before urine collection( Reference Zamora-Ros, Urpi-Sarda and Lamuela-Raventos 17 ). Overall, urinary resveratrol concentrations are adequate biomarkers of short-term and habitual dietary resveratrol intake in countries where wine is regularly consumed.
Our results may be influenced by measurement errors in the 24-HDR and DQ, which may attenuate our findings. However, the 24-HDR was collected using standardised software (EPIC-Soft) and administered in a face-to-face interview( Reference Slimani, Ferrari and Ocke 22 ). Furthermore, DQ were country-specific and previously validated for some resveratrol-rich foods such as wine( Reference Margetts and Pietinen 23 ). Second, estimation of dietary resveratrol intake may be limited by the incompleteness of Phenol-Explorer, although it is currently the most comprehensive database on polyphenols including stilbenes( Reference Neveu, Perez-Jimenez and Vos 5 ). Finally, the results might also be influenced by laboratory measurement error, although our analytical methodology has fulfilled all criteria to be validated( Reference Achaintre, Bulete and Cren-Olive 28 ).
In conclusion, urinary excretion of resveratrol was significantly and positively associated with resveratrol, stilbene and wine intakes estimated by 24-HDR and DQ. The correlation coefficients demonstrate that urinary resveratrol is an adequate biomarker of both acute and habitual resveratrol and wine intake in this population. Moreover, our results support the validity of our methodology, 24-HDR, DQ and the food composition database for estimating resveratrol intake. The findings of this study lay the groundwork for future studies to identify the role of resveratrol in the risk for chronic diseases.
Acknowledgements
The authors thank Mr Bertrand Hémon for his valuable help with the EPIC database.
This work was supported by the Institut National du Cancer, Paris (INCa grant 2011–105) and the Wereld Kanker Onderzoek Fonds (WCRF NL 2012/604). The national cohorts are supported by the French National Cancer Institute (L’Institut National du Cancer; grant 2009–139); Ligue contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (France); German Cancer Aid; German Cancer Research Center (DKFZ); German Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation (Greece); Italian Association for Research on Cancer; Compagnia San Paolo, (Italy); Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR) - Generalitat de Catalunya (exp. 2014 SGR 726), The Health Research Funds (RD12/0036/0018) (Spain). R. Z.-R. would like to thank the ‘Miguel Servet’ program (CP15/00100) from the Institute of Health Carlos III and European Social Fund.
R. Z.-R. and A. S. designed the research; R. Z.-R. wrote the manuscript; R. Z.-R. and J. A. R. analysed the data; J. A. R., D. A., A. S., P. F., M.-C. B.-R., F. R. M., A. A., T. K., V. K., H. B., S. K., A. T., P. L., C. L. V., D. P., P. C., S. P., R. T., F. R., H. N., H. F., I. R. reviewed and edited the manuscript; D. A. performed the laboratory analyses; A. S., P. F., M.-C. B.-R., F. R. M., A. A., T. K., V. K., H. B., S. K., A. T., P. L., C. L. V., D. P., P. C., S. P., R. T., F. R., H. N., H. F., I. R. contributed to the discussion. All authors have read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517001465







