Chronic kidney disease is becoming a worldwide disease. In adult patients, the proportion of chronic tubulointerstitial nephropathy (CTIN) is 2 % of native renal biopsies(Reference Baker and Pusey1) and it is up to 27 % in cases of chronic kidney disease of unknown aetiology(Reference Clarkson, Giblin and O’Connell2). Therefore, CTIN is one of the most common chronic kidney diseases. CTIN has many aetiologies, including virus, fungal infections, autoimmune and drug-related. And, the exposure of drug is the most common cause of CTIN(Reference Rossert3,Reference Perazella and Markowitz4) . In CTIN, renal interstitial fibrosis, tubular atrophy and irreversible injury of renal function can be observed(Reference Rossert3,Reference Perazella5) . It could finally cause renal failure and uraemia. In addition, it is difficult for clinicians to monitor tubulointerstitial nephropathy, especially in chronic cases. More than that, treatment for CTIN has multiple limits(Reference Joyce, Glasner and Ranganathan6). Adjuvant therapy through nutritional regulation has become a hot research topic at present.
Icariin (ICA) as an extraction of Chinese herbal medicine epimedium had wide pharmacological effects. It was widely utilised as antioxidant, anti-inflammation, anti-ageing actions and so on(Reference Li, Li and Mei7,Reference Song, Cai and Zhao8) . According to the statistics, ten studies have reported that ICA had effects on the renoprotective properties from 1946 to 2019(Reference Georgiadis, Zisis and Docea9). Ma et al.(Reference Pei Ma, Su and Qiu10) found that ICA could alleviate cisplatin-induced acute renal injury through disturbing apoptosis and NFκB activation in mice. And, Chen et al.(Reference Chen, Chen and Guan11) found that the application of 20 mg/kg per d ICA could possess antifibrotic and anti-inflammatory properties. ICA attenuated the enhancement of creatinine (Cr), blood urea nitrogen (BUN) and the expression of transforming growth factor-beta 1 (TGF-β1) and collagen which occurred in rats with diabetic nephropathy(Reference Qia, Kai-Chena and Sua12). It was also verified that ICA had significant protective effects in other diseases, such as IgA nephropathy(Reference Zu, Mu and Li13–Reference Zhang, Wang and Li16). Whereas diabetic nephropathy is one of the complications of diabetic systemic microangiopathy and IgA, nephropathy is the most common primary glomerular disease. The two kinds of kidney disease are different from chronic tubulointerstitial toxicity. On the other hand, Se is an essential trace element for human and animals. It exists in two forms: inorganic Se and organic Se. Organic Se generally exists in the form of selenomethionine (SeMet), which is safer and less toxic than inorganic Se. What is more, organic Se is more easily to be absorbed by human and animals as compared with inorganic Se(Reference Kieliszek17). It has effects of antioxidant and lightening nephrotoxicity(Reference Ananth, Miyauchi and Thangaraju18,Reference Liu, Dong and Yang19) . Our laboratory has been conducting research on organic Se for a long time, among which the development of Se-rich probiotics successfully obtained the Chinese invention patent, and the main component of Se-rich probiotics is SeMet. Previous studies have proved that SeMet can reduce ochratoxin A (OTA)-induced nephrotoxicity by increasing selenoenzyme expression and can alleviate T-2 toxin-induced oxidative damage and inflammatory reaction of rabbit renal(Reference Gan, Xue and Huang20,Reference Liu, Dong and Yang21) . However, whether the presence of SeMet can enhance the renal protective effects of ICA has not been studied, which is also a popularisation and application of Se-rich probiotics in adjuvant therapy of nephropathy. This research hopes to provide a foundation for the development and application of the adjuvant therapy of nephropathy.
A long-time use of cyclosporine A (CsA) can induce chronic tubulointerstitial nephrotoxicity(Reference Myers, Ross and Newton22,Reference Mattos, Olyaei and Bennett23) . Therefore, it was employed to develop a chronic CsA nephropathy model, but there are many adverse effects in this model(Reference Gillum, Truong and Tasby24–Reference Young, Burdmann and Johnson27). OTA is a mycotoxin produced by Aspergillus and Penicillium, and its exposure as well as CsA exposure can induce nephrotoxicity(Reference Ciarcia, Damiano and Squillacioti28,Reference Li, Chen and Zheng29) . It often contaminates human food and animals feed and accumulates in the kidney(Reference Liang, Huang and Luo30,Reference Gareis and Scheuer31) . To investigate the combined nephroprotective effects of ICA and SeMet, the novel CTIN model developed by low dosage of CsA and nontoxic dosage of OTA in our previous study(Reference Hou, Le and Lin32) was employed in the present study.
Toll-like receptor 4 (TLR4) as one of the innate families is related to renal fibrosis. Its expression could be up-regulated after the unilateral ureteral obstruction with activating NFκB signalling pathway in mice(Reference Chen, Sha and Li33). It was also reported that kidney pericytes triggered the activation of TLR4/MyD88 signalling in response to renal tubular injury(Reference Nakagawa34). Therefore, our objectives are to investigate the combined protective effects of ICA and SeMet by employing the novel CTIN models induced by CsA and OTA and explore the underlying mechanism in vivo and in vitro.
Methods
Materials
ICA (Purity: 99·05 %, S2312) and CsA (Purity: 99·66 %, S2286) were bought from Selleckchem Company. SeMet (1611955) and OTA (Purity: ≥98 %, O1877) were bought from Sigma-Aldrich.
Experimental animals
C57BL/6 male mice, 6–8 weeks old, were purchased from the Center of Laboratory Animals, Yangzhou University (Yangzhou, China). The mice were housed in cages in animal quarters under constant conditions of 12 h light–12 h dark cycle, temperature (23 (sd 1) °C) and humidity (60 (sd 10) %) for 1 week prior to experiment and had free access to food (standard chow diet) and tap water. All procedures involving mice were carried out in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Bethesda, MD, USA), approved by the Committee for the Care and Use of Experimental Animals at the Agriculture University of Nanjing (Certification No.: SYXK (Su)2011–0036).
Treatments of animals
Thirty mice were randomly divided into five groups (a: control; b: CTIN model; c: CTIN model + 20 mg/kg per d ICA; d: CTIN model + 0·4 mg/kg per d SeMet; e: CTIN model + ICA + SeMet)(Reference Liu, Dong and Yang21,Reference Chen, Chen and Guan35,Reference Kumbhar, Khan and Parveen36) . Each group had six mice. Mice were treated with CsA and OTA (for 28 d) with or without ICA or/and SeMet (for another 14 d). At the day after last day, all mice were killed for collecting serum, urine and kidney tissue.
Renal function analysis
Blood was collected from the eyeballs of mice and centrifuged at 1000 rpm for 10 min to extract serum. BUN and serum Cr in serum were detected by using standard commercial kits (Jiancheng) based on the manufacturer’s protocol. Urine gravity and urine protein were measured by a handheld refractometer (SUR-NE).
Histopathological and immunohistochemical analyses
Kidneys were collected and fixed in 10 % neutral-buffered formalin, sectioned at a 4 μm thickness and processed for paraffin embedding. Haematoxylin–eosin, Masson and immunohistochemical staining were used according to standard procedures (Servicebio). It was observed by optical microscopy (Nikon Instruments, Inc.) to detect the histopathological changes.
Cell culture and treatment
Human renal proximal tubule epithelial (HK-2) cells were purchased from Gefan Biotechnology and cultured in the RPMI-1640 medium (Gibco). Heat-inactivated 10 % fetal bovine serum (Gibco), penicillin (100 U/ml) and streptomycin (100 U/ml) were added into the medium. Cells were exposed to combined toxins of CsA and OTA and then treated with or without ICA or/and SeMet.
Cell viability assay
Cells were seeded at a density of 4 × 103 cells/well in the ninety-six-well plates and exposed to CsA and OTA in combination for 48 h. Three to four hours before the end of the processing, 15 μl 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (5 mg/ml) was added into the culture medium at 37°C. Then, the supernatants were removed, and 150 μl dimethyl sulfoxide was added into the wells to dissolve the crystal. The absorbance was detected at 490 nm with a secondary wavelength of 655 nm by using a Microplate Reader (Thermo Fisher). The OD value of each group was obtained and expressed as percentages of control group. Six replications were performed.
Cell proliferation assay
Cells were cultured with corresponding treatment. After the treatment, cells were incubated with EdU markers (10 μm) for 2 h and fixed by 4 % paraformaldehyde for 15 min. Then, cells were washed, penetrated and stained according to the protocol. A BeyoClick ™ EdU cell proliferation test kit purchased from Beyotime Biotechnology was used to evaluate cell proliferation ability. The results were scanned with an ordinary optical microscope (Nikon Instruments, Inc.).
Cell apoptosis assay
To investigate nuclear morphology, cells were cultured on 20 mm round coverslips (WHB) in twelve-well plates. After similar treatments, the slides were washed three times with PBS. Cells were then stained with Hoechst33258 (1 mg/ml) for 10 min. Finally, the slides were washed again and scanned with a fluorescence microscope.
Real-time PCR analysis
After corresponding treatment, total RNA was extracted by using the RNAiso Plus kit (TAKARA) according to the protocol. Total RNA (500 ng) was reversely transcribed to single-stranded complementary DNA. The relative mRNA levels were detected by the Δ cycle threshold method, with β-actin serving as the housekeeping gene. Real-time PCR was performed via using SYBR Premix Ex Taq II (TAKARA) and ABI Step one real-time PCR system (Applied Biosystems). The primers (Table 1) were designed and synthesised by Sangon Biotech.
Table 1. List of genes with their forward and reverse primer sequences used for the gene expression analysis by real-time PCR
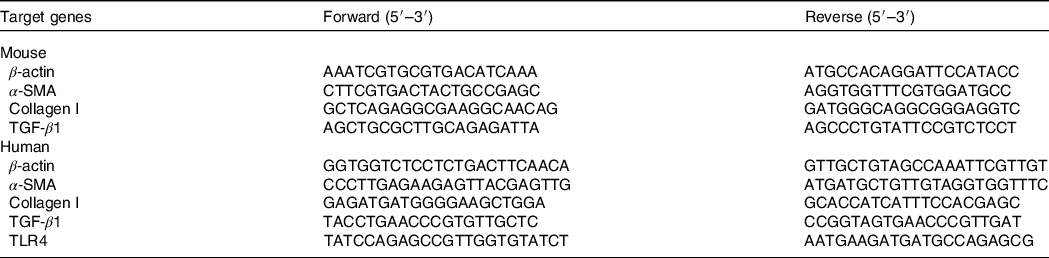
α-SMA, α-smooth muscle actin; TGF-β1, transforming growth factor-beta 1; TLR4, toll-like receptor 4.
Western blotting analysis
The total protein was extracted, and their concentrations were measured by BCA protein assay kits (Beyotime). Forty micrograms of protein was denatured in loading buffer and heated at 95°C for 5 min. By using 12 % SDS-PAGE assay, the protein was transferred to polyvinylidene difluoride membranes. The membranes were incubated with specific primary antibodies including anti-α-smooth muscle actin (α-SMA) (Bioss), anti-TGF-β1 (ABclone), anti-TLR4, anti-IκBα, anti-p-IκBα (Santa), anti-NFκB anti-p-NFκB and anti-β-actin (CST) at 4°C overnight. The polyvinylidene difluoride membranes were washed with TRIS-buffered saline three times and then incubated with HRP-labelled anti-rabbit secondary antibody. Blots were visualised and analysed by a Luminescent Image Analyzer (FUJIFILM 171 LAS-4000). The results were expressed as percentage with respect to the control group.
Immunofluorescence assay by laser scanning confocal microscope
To analyse NFκB foci, the slides were washed three times with PBS after corresponding treatment and incubated in specific primary antibodies (anti-NFκB; CST). Then, slides were washed again and incubated in fluorescein isothiocyanate-labelled anti-rabbit IgG antibody (Beyotime) and DAPI (Solarbio) for 1 h 20 min. Green fluorescent proteins NFκB were visualised via a laser scanning confocal microscope (Zeiss LSM 710 META confocal system).
Construction of toll-like receptor 4 overexpression plasmid (pcDNA3.1-toll-like receptor 4 recombinant plasmid)
A specific primer of TLR4 was designed by SnapGene Software 2.3.2 according to the nucleotide sequence of human TLR4 (GeneBank: AB445638.1). The restriction enzyme sites were inserted into forward primer (BamH I at the 5′ end) and the reverse primer (Xho I at the 3′ end) to achieve subcloning of the amplified 2520-bp fragment. The forward primer is 5′-CGCGGATCCATGATGTCTGCCTCGCGCCTG-3′, and the reverse primer is 5′-CCGCTCGAGTCAGATAGATGTTGCTTCCTGCCAAT-3′. First-strand complementary DNA was synthesised from extracted RNA, and normal PCR was carried out by the ABI Prism (Applied Biosystems). The purified PCR product and plasmid pcDNA3.1(-) vector were digested with Xho I and BamH I. The digested PCR product and vector were purified and connected with T4 ligase to construct the pcDNA3.1-TLR4 recombinant plasmid (pc-TLR4), and it was verified.
Transient transfection of pcDNA3.1-toll-like receptor 4 recombinant plasmid in human renal proximal tubule epithelial cells
Via using X-tremeGENE transfection reagent (Roche), pc-TLR4 was transfected into HK-2 cells cultured in the RPMI-1640 medium (Gibco) supplemented with 10 % fetal bovine serum and 1 % penicillin (100 U/ml) and streptomycin (100 U/ml). Control cells were prepared by transfecting with the empty pcDNA3.1, and the other groups were with corresponding treatment. Relative TLR4 mRNA levels in transfected HK-2 cells were analysed by real-time PCR, and TLR4 protein expression was analysed by Western blot.
Statistical analysis
Results were statistically analysed by one-way ANOVA, followed by Duncan’s multiple range tests to analyse the means. All statistical analyses were performed with the help of SPSS 18.0 and GraphPad Prism 7.0. The sample was determined using G* power 3.1.9.7 (power 80 %, effect size 0·8 and 5 groups), and estimated six mice were required per group. Each experiment was performed at least three times, and the values were presented as mean and standard deviation. Statistical significance was set at P < 0·05 and P < 0·001.
Results
Protective effects of icariin or/and selenomethionine on renal function and fibrosis in chronic tubulointerstitial nephropathy model mice
To detect the protective effects of ICA or/and SeMet on renal function and fibrosis in model mice, we tested kidney index, urine protein, urine gravity, BUN, serum Cr, kidney pathological changes and expression of renal fibrosis-related genes and proteins. As shown in Fig. 1, urine protein, urine gravity, BUN, serum Cr and expression of renal fibrosis-related genes (TGF-β1, α-SMA and collagen I) were much higher in the model group than that in the control group (P < 0·05). Kidney index was significantly decreased in the model group (P < 0·05). Fortunately, these changes were reversed by employing ICA or/and SeMet, especially the combination of them. Meanwhile, kidney pathology revealed atrophy of glomeruli, epithelial exfoliation of renal tubules and infiltration of inflammatory cell in the model group (b), while ICA or/and SeMet (c, d, e) obviously improved the histopathological damage in Fig. 1E. The results of Masson staining demonstrated that collagen I deposition which was stained by blue was relieved by ICA or/and SeMet and the combination of them exhibited better protection than single usage of ICA or SeMet (P < 0·05). The up-regulated gene expression of TGF-β1, α-SMA and collagen I induced by CsA and OTA was reversed more significantly in the combined group of ICA and SeMet than that in the single ICA or SeMet group (Fig. 1F–H). In Fig. 1I, IHC of α-SMA was observed and increasing expression of renal fibrosis-related proteins (TGF-β1 and α-SMA) induced by CsA and OTA was markedly decreased by treatment of ICA or/and SeMet (Fig. 1J–L). These results suggested that ICA and SeMet could improve renal fibrosis, renal dysfunction and renal pathological changes in CTIN model mice and combined effects of them were better than alone.
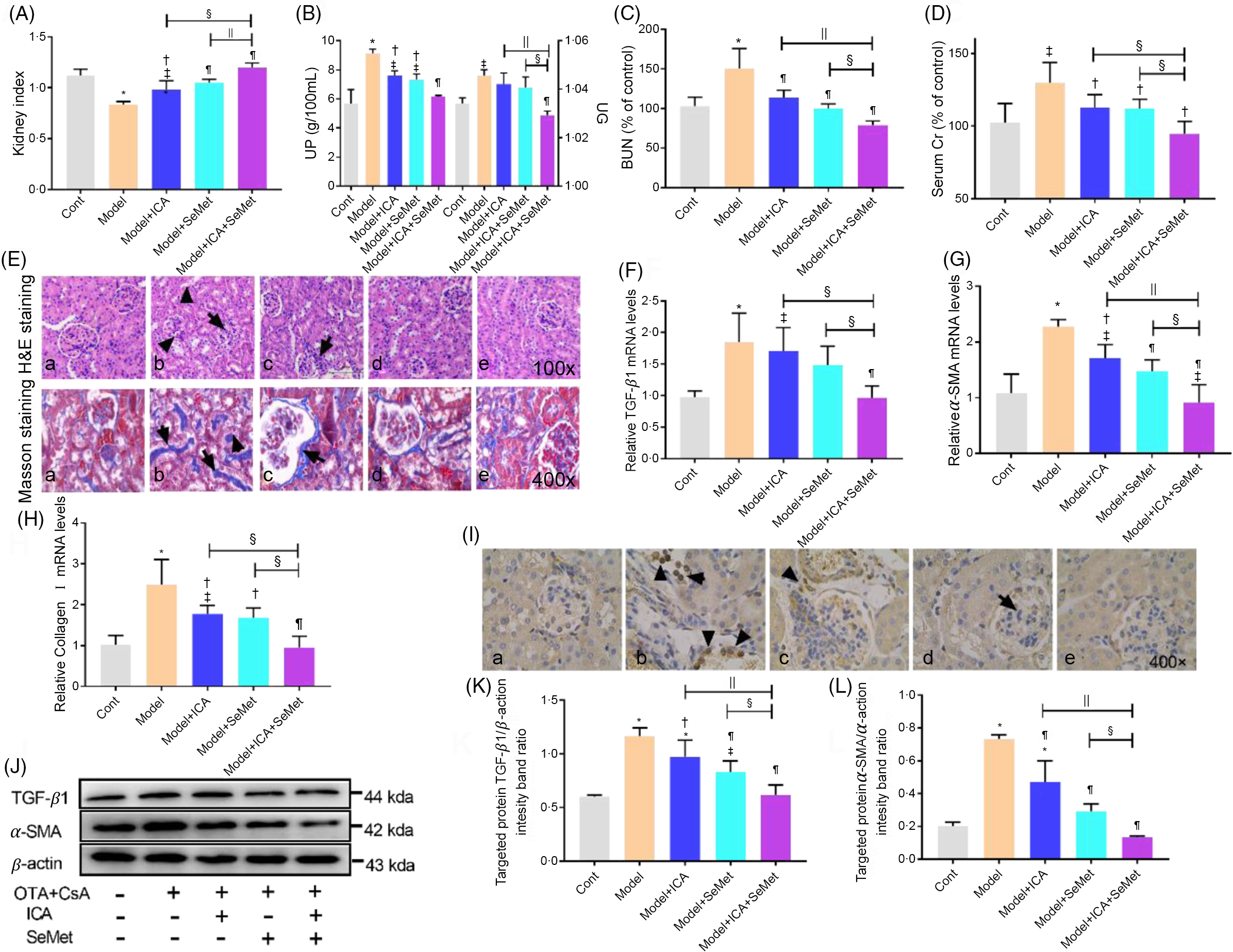
Fig. 1. Protective effects of icariin (ICA) or/and selenomethionine (SeMet) on renal function and fibrosis in chronic tubulointerstitial nephropathy (CTIN) model mice. Kidney index (A), urine protein (UP), urine gravity (UG) (B), Serum blood urea nitrogen (BUN) (C) and serum creatinine (Scr) (D) were assayed. Haematoxylin–eosin (H&E) (E, 100×), Masson staining (E, 400×), transforming growth factor-beta 1 (TGF-β1) (F, J, K), α-smooth muscle actin (α-SMA) (G, J, L) and collagen I (H) mRNA and protein levels were detected as described in the Materials and Methods (a: control; b: model; c: model + ICA; d: model + SeMet; e: model + ICA + SeMet). Immunohistochemical staining of α-SMA (I) was observed. Data were presented as means and standard deviations (n 6 or n 3). Compared with control group, ‡ P < 0·05 was considered statistically significant and * P < 0·01 was considered strongly significant. Compared with model group, † P < 0·05 was considered statistically significant and ¶ P < 0·01 was considered strongly significant. Compared with model + ICA + SeMet group, § P < 0·05 was considered statistically significant and || P < 0·01 was considered strongly significant.
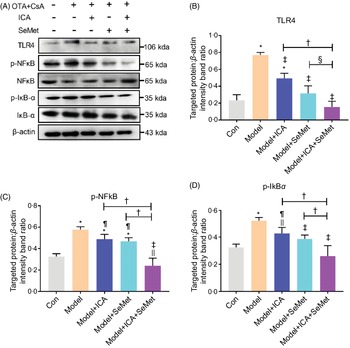
Effects of icariin or/and selenomethionine on the protein expression of toll-like receptor 4/NFκB signalling pathway in chronic tubulointerstitial nephropathy model mice
NFκB, as a downstream protein of TLR4, would be phosphorylated and transported into cell nucleus when it was activated. IκBα would also be phosphorylated and then degraded. Protein expressions of TLR4, p-NFκB and p-IκBα were significantly up-regulated in the CTIN model mice (Fig. 2). To investigate the effects of ICA or/and SeMet on expression of TLR4 and NFκB in CTIN model mice, target proteins/β-actin intensity band ratios were measured. The up-regulated expression of TLR4, p-NFκB and p-IκBα induced by CsA and OTA was significantly decreased by the treatment of ICA or/and SeMet as shown in Fig. 2B–D (P < 0·05). Treatment of ICA and SeMet in combination obviously worked better (P < 0·05). It suggested that the application of ICA and SeMet could activate TLR4/NFκB signalling pathway in CTIN model mice.
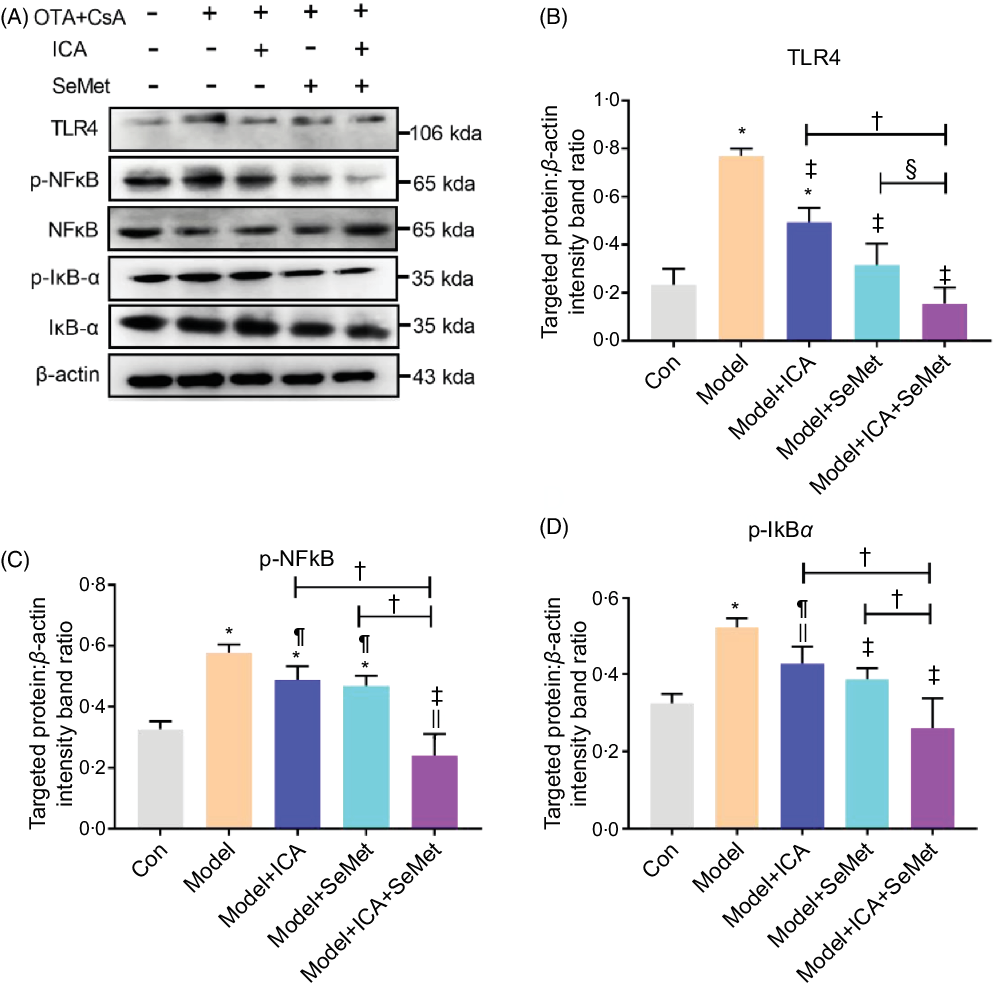
Fig. 2. Effects of icariin (ICA) or/and selenomethionine (SeMet) on the protein expression of toll-like receptor 4 (TLR4)/NFκB signalling pathway in chronic tubulointerstitial nephropathy (CTIN) model mice. TLR4 (A, B), NFκB (A, C) and IκBα (A, D) were detected as described in the Materials and Methods. Data were presented as means and standard deviations (n 3). Compared with control group, || P < 0·05 was considered statistically significant, and * P < 0·01 was considered strongly significant. Compared with model group, ¶ P < 0·05 was considered statistically significant and ‡ P < 0·01 was considered strongly significant. Compared with model + ICA + SeMet group, § P < 0·05 was considered statistically significant and † P < 0·01 was considered strongly significant.
Protective effects of icariin or/and selenomethionine on renal cytotoxicity and fibrosis in human renal proximal tubule epithelial cells
Cell viability was measured to select the appropriate concentrations of ICA and SeMet. As shown in Fig. 3A and B, HK-2 cells were incubated with 0, 1, 2, 4, 8, 16 or 32 µg/ml ICA or 0, 1, 2, 4, 8, 16 or 32 µm SeMet for 48 h. The results showed that cell viability was significantly increased at 2 µg/ml ICA or 2 µm SeMet. And, the combined effect of ICA and SeMet on cell viability was also determined by 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The combination of 2 µg/ml ICA and 2 µm SeMet performed the best protective effects (Fig. 3C), so that it was selected in subsequent investigation. In the presence of 6 µg/ml CsA and 0·5 µg/ml OTA in combination, the decreasing cell viability was markedly improved by ICA or/and SeMet, especially combination of them (P < 0·01) in Fig. 3D. Nuclear apoptosis and cell proliferation ability were observed by Hoechst33258 and EdU markers staining. In Fig. 3E and F, the decreasing trend of cell proliferation ability and the increasing trend of nuclear apoptosis were counteracted by ICA or/and SeMet. What is more, the expression of renal fibrosis-related genes was measured by RT-PCR. As shown in Fig. 3G–I, the upward trends of TGF-β1 and α-SMA were reversed by ICA or/and SeMet (P < 0·05). These results indicated that ICA and SeMet could ameliorated renal cytotoxicity and fibrosis in vitro and the combined effects of them were better.
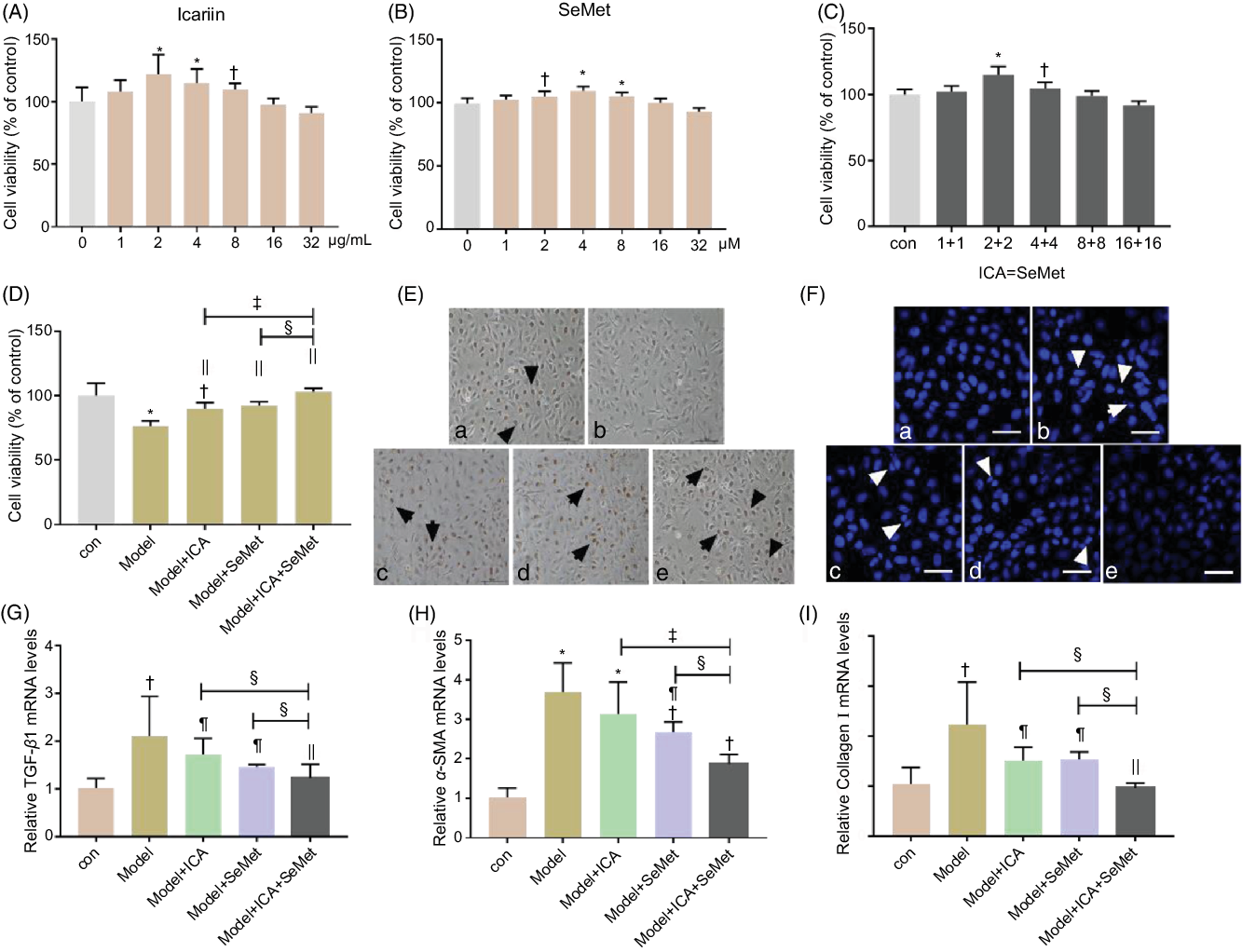
Fig. 3. Protective effects of icariin (ICA) or/and selenomethionine (SeMet) on renal cytotoxicity and fibrosis in human renal proximal tubule epithelial (HK-2) cells. Cell viability (A, B, C, D), proliferation (E, 100×) and apoptosis (F, 400×) were detected; transforming growth factor-beta 1 (TGF-β1) (G), α-smooth muscle actin (α-SMA) (H) and collagen I (I) mRNA levels were measured as described in the Materials and Methods (a: control; b: model; c: model + ICA; d: model + SeMet; e: model + ICA + SeMet). Data were presented as means and standard deviations (n 5 or n 3). Compared with control group, † P < 0·05 was considered statistically significant and * P < 0·01 was considered strongly significant. Compared with model group, ¶ P < 0·05 was considered statistically significant and || P < 0·01 was considered strongly significant. Compared with model + ICA + SeMet group, § P < 0·05 was considered statistically significant and ‡ P < 0·01 was considered strongly significant.
Effects of icariin or/and selenomethionine on the protein expression of toll-like receptor 4/NFκB signalling pathway in human renal proximal tubule epithelial cells
In the presence of CsA and OTA in combination, HK-2 cells were incubated with ICA or/and SeMet. The protein expression of TLR4, p-NFκB and p-IκBα was measured. As shown in Fig. 4A–D, the expression of them was sharply elevated in model group (P < 0·01). Supplement with ICA or/and SeMet significantly lessened the uptrend expression of TLR4, p-NFκB and p-IκBα. The combination of ICA and SeMet was more effective. In addition, the location of NFκB was detected and it was transported into the nucleus in the model group, while ICA and SeMet inhibited the NFκB activation and its transportation to the nucleus (Fig. 4E). Treatment of ICA and SeMet in combination obviously worked better. These results further verified that combination of ICA and SeMet could reduce the protein expression of TLR4/NFκB signalling pathway, which could play a key role in resisting the renal injury.
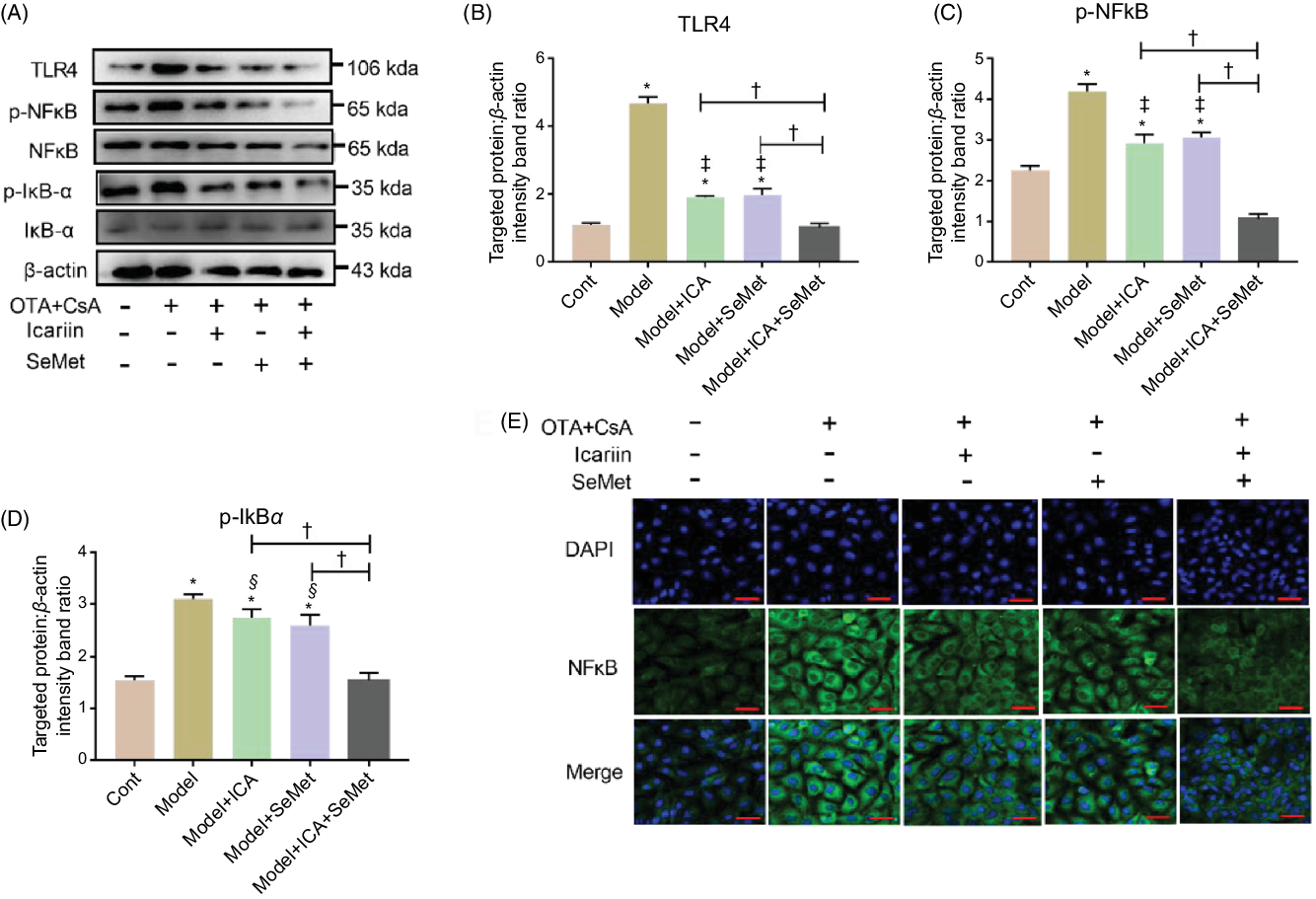
Fig. 4. Effects of icariin (ICA) or/and selenomethionine (SeMet) on the protein expression of toll-like receptor 4 (TLR4)/NFκB signalling pathway in human renal proximal tubule epithelial (HK-2) cells. TLR4 (A, B), p-NFκB (A, C), p-IκBα (A, D) and NFκB foci (E, 400×) were observed by Western blot and indirect immunofluorescence. Compared with control group, * P < 0·01 was considered strongly significant. Data were presented as means and standard deviations (n 3). Compared with model group, § P < 0·05 was considered statistically significant and ‡ P < 0·01 was considered strongly significant. Compared with model + ICA + SeMet group, † P < 0·01 was considered strongly significant.
Construction and transient transfection of pcDNA3.1-toll-like receptor 4 recombinant plasmid in human renal proximal tubule epithelial cells
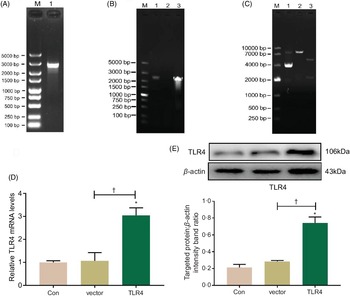
Total RNA was extracted from HK-2 cells and reversely transcribed into complementary DNA, which was then amplified with PCR using a TLR4 primer; PCR product was authenticated by gel electrophoresis, and it showed that the product was a single target TLR4 gene, 2520 bp in length (Fig. 5A). The eukaryotic TLR4 overexpression plasmid (pc-TLR4) constructed using a pcDNA3.1 vector was then authenticated by colony PCR (Fig. 5B), double restriction endonucleases digestion and DNA sequencing (Fig. 5C). The human pc-TLR4 reconstructive plasmid was transiently transfected into HK-2 cells, and it led to overexpression of TLR4. In Fig. 5D and E, the mRNA and protein expression of TLR4 were significantly increased compared with control and empty vector-transfected groups (P < 0·01).
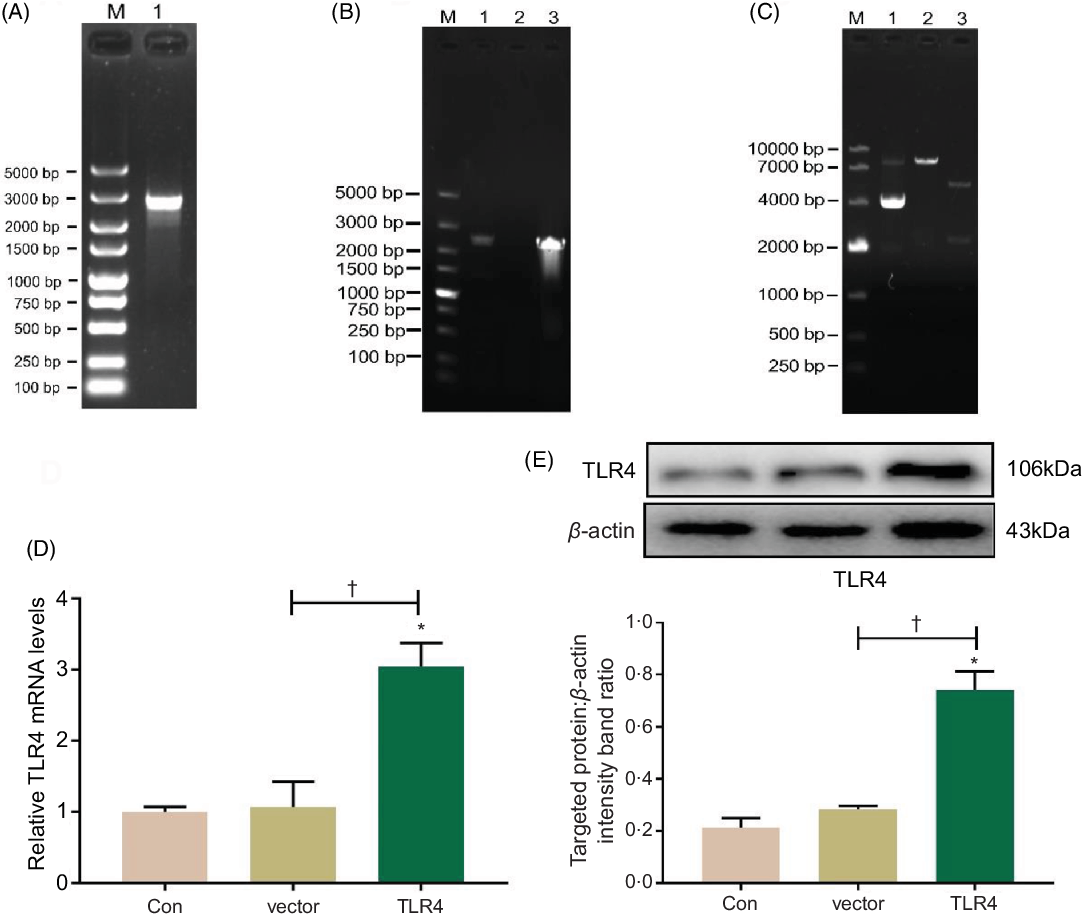
Fig. 5. Construction and transient transfection of pc-toll-like receptor 4 (TLR4) in human renal proximal tubule epithelial (HK-2) cells. A single target TLR4 gene, 2520 bp in length, was identified using PCR and electrophoresis (a). pcDNA3.1-TLR4 was verified by colony PCR (B) and restriction endonuclease digestion (C). The expression of TLR4 was measured by quantitative real-time PCR (qRT-PCR) (D) and Western blot (E). Data were presented as means and standard deviations (n 3). Compared with control group, * P < 0·01 was considered strongly significant. Compared with vector group, † P < 0·01 was considered strongly significant.
Effects of toll-like receptor 4 overexpression on nephroprotection of icariin and selenomethionine in combination in human renal proximal tubule epithelial cells
To determine whether TLR4 overexpression depresses the nephroprotection of ICA and SeMet in combination in HK-2 cells, we investigated the mRNA and protein expression which was related to renal fibrosis and TLR4/NFκB signalling pathway. As shown in Fig. 6A–C, the downtrend of cell viability and relative mRNA levels of TGF-β and α-SMA were prominently reversed by pc-TLR4 (P < 0·05). The results of Western blot showed similar changes in Fig. 6D–F. Furthermore, downstream proteins (NFκB and IκBα) of TLR4 were detected by Western blot. As shown in Fig. 6D, G and H, the phosphorylation of NFκB and IκBα was lessened after treatment with the combination of ICA and SeMet, while it was markedly increased due to supplementary of pc-TLR4. And, location of NFκB was visualised by a laser scanning confocal microscope in Fig. 6I. It was showed that NFκB was obviously activated and it was translocated into the nucleus due to the treatment of pc-TLR4. These results manifested that combination of ICA and SeMet could protect against chronic kidney disease induced by CsA and OTA through blocking TLR/NFκB signalling pathway.
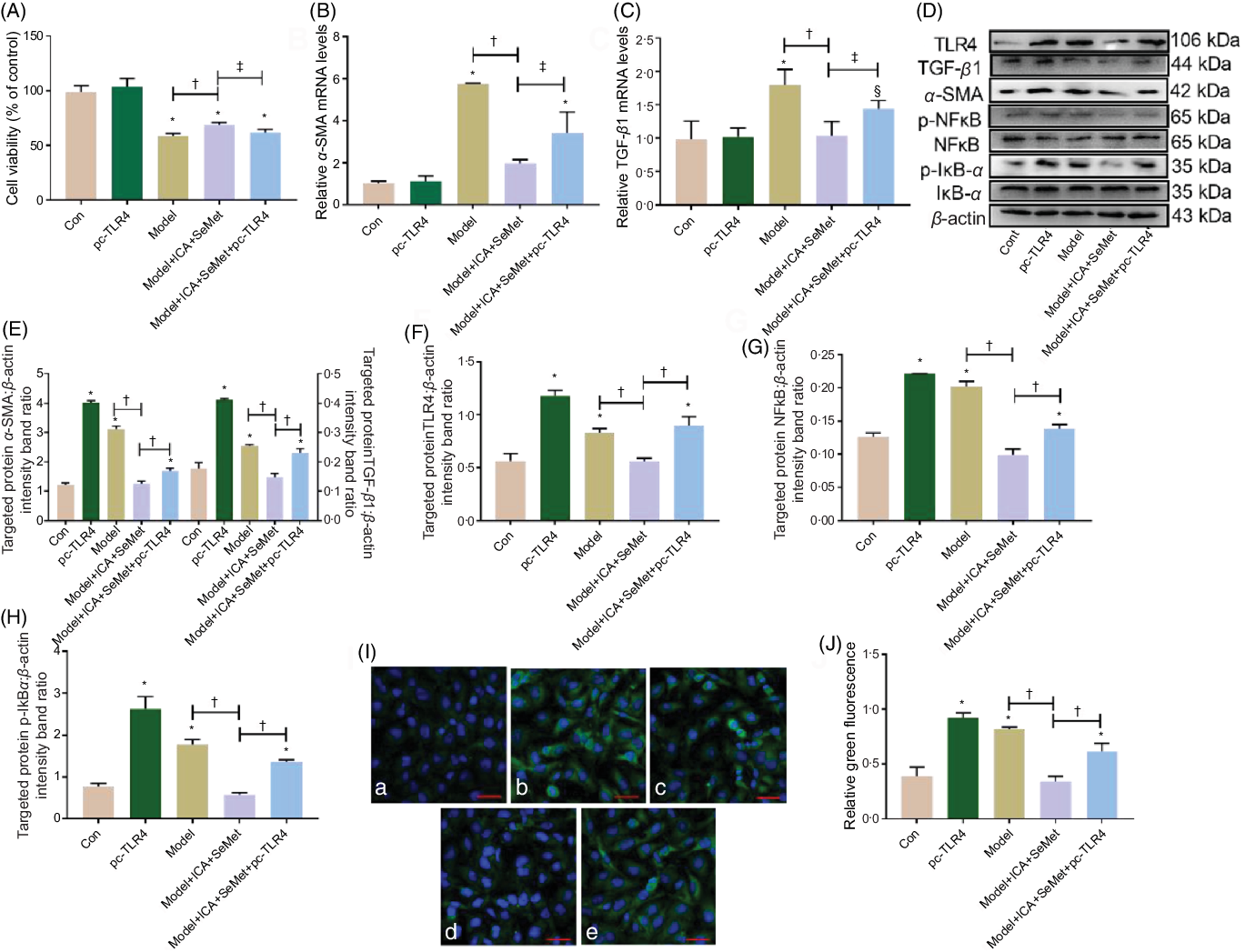
Fig. 6. Effects of toll-like receptor 4 (TLR4) overexpression on nephroprotection of icariin (ICA) and selenomethionine (SeMet) in combination in human renal proximal tubule epithelial (HK-2) cells. Cell viability (A), relative mRNA levels of α-smooth muscle actin (α-SMA) (B) and transforming growth factor-beta 1 (TGF-β1) (C), protein expression of TGF-β1, α-SMA (D, E), TLR4 (D, F), p-NFκB (D, G), p-IκBα (D, H) and NFκB foci (I, J) were assessed (a: control; b: pc-TLR4; c: model; d: model + ICA + SeMet; e: model + ICA + SeMet + pc-TLR4). Data were presented as means and standard deviations (n 6 or n 3). Compared with control group, § P < 0·05 was considered statistically significant and * P < 0·01 was considered strongly significant. ‡ P < 0·05 was considered statistically significant, and † P < 0·01 was considered strongly significant.
Discussion
CTIN is characterised by tubulointerstitial fibrosis, and it often obtains a delayed diagnosis due to its non-specific presenting signs and symptoms. ICA has been widely used as an effective invigorating kidney and strengthening yang component of Chinese herbal medicine. It has been employed to protect against different kidney diseases(Reference Pei Ma, Su and Qiu10,Reference Zhang, Wang and Li16,Reference Qia, Kai-Chena and Liub37) . And, SeMet as the main source of organic Se was found that it could alleviate cisplatin-induced and OTA-induced nephrotoxicity(Reference Gan, Xue and Huang20,Reference Sar, Montes-Bayo and Blanco González38) . More than that, it was also reported that SeMet could improve cyclophosphamide-induced kidney toxicity(Reference Ayhanci, Günes and Sahinturk39). However, the nutritional regulation effects of ICA and SeMet in combination on CTIN and the specific mechanism are unknown. In our previous study, a novel and positive CTIN model was developed by non-toxic OTA and low-dosage CsA in combination and it alleviated the side effects of high-dosage CsA(Reference Hou, Le and Lin32). Therefore, the present nephropathy model induced by CsA and OTA was implemented for evaluating the nephroprotective effects of ICA or/and SeMet on it. Fortunately, the results showed that ICA or/and SeMet could ameliorate renal pathology damage, renal dysfunction, renal fibrosis in our CTIN model mice and HK-2 cells and the combination of them performed better than alone in most indexes including renal fibrosis.
Chronic kidney disease will eventually lead to end-stage kidney disease. It was characterised by continuous renal damage, dysfunction, glomerulus and tubular fibrosis, especially the sustaining accumulation of extracellular matrix(Reference Stroo, Emal and Butter40). What is more, the overproduction of TGF-β and α-SMA plays a key role in the process of renal fibrosis(Reference Iekushi, Taniyama and Azuma41–Reference Koh, Kim and Kim44). It was reported that ICA or SeMet, respectively, ameliorated the cisplatin-induced nephrotoxicity through inhibiting oxidative stress, inflammation and apoptosis(Reference Pei Ma, Su and Qiu10,Reference Sar, Montes-Bayo and Blanco González38) . In the present study, on the one hand, renal pathology damage induced by CsA and OTA was ameliorated by ICA or/and SeMet and we also found that they decreased the expression of collagen I, renal fibrosis-related indexes of TGF-β and α-SMA which were up-regulated in CTIN model mice and cells. On the other hand, the decreasing kidney indexes and the increasing indexes of urine protein, urine gravity, BUN and serum Cr were significantly reversed by ICA or/and SeMet in model mice. And, the combination of them performed better. It was consistent with the previous reports that ICA or SeMet had protective effects on renal fibrosis and impaired kidney function(Reference Li, Wang and Chu45–Reference Zhang, Yuan and Xu47).
TLR4 can regulate innate immune response through triggering TRIF, TRAM and MyD88 which facilitate the activation of NFκB including the phosphorylation of p65 and its translocation into the nucleus(Reference Fitzgerald, Rowe and Barnes48,Reference Xu, Hao and Gan49) . TLR4 plays a key role in the progress of renal and liver fibrosis. It was reported that activation of TLR4-MyD88-NFκB axis could enhance TGF-β signalling and induce hepatic fibrosis(Reference Seki, De Minicis and Osterreicher50). Our previous study also investigated that Lycium barbarum polysaccharides could lessen CCl4-induced oxidative stress, inflammatory and liver fibrosis via TLR4/MyD88/NFκB signalling pathway in rats(Reference Gan, Liu and Liu51). In addition, it was also reported that TLR4 mutant mice which had a missense point mutation to make TLR4 receptor non-functional showed less renal fibrosis and progression of chronic kidney disease compared with wide-type mice(Reference Souza, Tsuji and Baranova52). In chronic kidney disease, kidney damaged cells release factors which are recognised by pattern recognition receptors including TLR and NOD-like receptors(Reference Kurts, Panzer and Anders53). It means that TLR4 is closely related to chronic kidney disease. ICT, a derive of ICA, could attenuate LPS-induced inflammation via inhibition of CD14/TLR4 signalling pathway in human monocytes(Reference Wu, Zhou and Chen54). It was consistent with the results of our present study. We found that the expression of TLR4, p-NFκB and p-IκBα was significantly augmented in CTIN model, while the uptrend was down-regulated after the administration of ICA or/and SeMet in vivo and in vitro. The NFκB translocation was also impacted. To further verify it, pc-TLR4 overexpression plasmid was constructed in vitro. The combined nephroprotective effects of ICA and SeMet were inhibited by overexpression of TLR4. Therefore, we concluded that the combined nephroprotective effects of ICA and SeMet on alleviating chronic tubulointerstitial injury induced by CsA and OTA were through blocking TLR4/NFκB signalling pathway.
In conclusion, the present study demonstrated that ICA or/and SeMet could lessen the renal dysfunction, pathological lesion, cell apoptosis and tubulointerstitial fibrosis in CTIN model induced by non-toxic OTA and low dosage of CsA and the combination of ICA and SeMet showed better protective effects than single ICA or SeMet. It was proved that the combined nephroprotection of ICA and SeMet performed its protective effects by blocking the TLR4/NFκB signalling pathway. It will provide novel insight into exploring an adjuvant therapeutic orientation for nutritionally regulating chronic tubulointerstitial injury.
Acknowledgements
We thank the support by the National Natural Science Foundation of China (32072926 and 31772811); Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_0577) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Jiangsu, China).
All the authors declared no competing interests.
K. H.: Conceptualisation, project administration, funding acquisition and supervision; L. H.: data curation, formal analysis, investigation, resources, roles/writing – original draft and writing – review & editing; Z. L.: formal analysis and resources; A. X and G. L.: investigation and resources; L. G.: resources and software; S. L.: resources; A. M.: writing – review & editing; F. G.: review.