The pandemic (H1N1) 2009 (pH1N1) virus emerged in North America in April 2009 [1, 2] and spread rapidly around the world. The World Health Organization (WHO) declared the start of the 2009 influenza pandemic on 11 June 2009 [3]. In Hong Kong, the first imported case of pH1N1 infection was detected on 1 May 2009. Stringent public health containment measures were implemented in order to delay community transmission of the virus. These included isolation of confirmed and suspected cases, quarantine of close contacts and oseltamivir prophylaxis for those who were possibly exposed.
The first locally acquired pH1N1 infection (without identifiable epidemiological linkage to an imported case) was detected on 10 June 2009. Surveillance for locally acquired pH1N1 infections was subsequently enhanced by the activation of designated influenza clinics on 12 June 2009. Patients with symptoms of influenza-like illness were advised to attend these clinics and respiratory specimens were collected for pH1N1 virus testing, using reverse transcription–polymerase chain reaction (RT–PCR) and viral culture. More than 400 laboratory tests for pH1N1 virus were performed every day at that time.
On 16 June 2009, a case of pH1N1 infection was confirmed in an eighth-grade student of a secondary school (school X). He developed fever, cough, sore throat and runny nose on 14 June 2009. Initial investigation revealed that school X had an increase in absenteeism on 16 June 2009. On 17 June 2009, 25 additional students of the school were confirmed as having pH1N1 infection. We carried out an epidemiological investigation to delineate the characteristics of the outbreak, gauge the extent of secondary household transmission and assess the protective role of oseltamivir in household contacts.
We advised students and staff who had fever or respiratory symptoms to seek medical attention at designated influenza clinics where respiratory specimens were collected. Those who were tested positive for pH1N1 virus by RT–PCR or viral culture were interviewed by telephone using a standardized questionnaire. Their household contacts were interviewed for symptoms of pH1N1 infection and were offered oseltamivir prophylaxis. They were asked to inform us if they developed fever or respiratory symptoms during the 2 weeks after the last contact with the index case in their family. Symptomatic household contacts were seen at designated influenza clinics or hospitals for medical consultation and respiratory specimens were collected for pH1N1 testing. A confirmed case of pH1N1 infection in this outbreak was defined as any student, school staff, or their household contacts who had onset of fever and/or respiratory symptoms (cough, runny nose or sore throat) between 5 and 30 June 2009, and had a respiratory specimen test positive for pH1N1 virus by RT–PCR or viral culture.
For household clusters, the confirmed case with the earliest onset date was considered the index case. All other symptomatic and laboratory-confirmed cases in the family were considered secondary cases. In this study, we assumed that all secondary cases contracted the infection from their household index case. We computed the secondary household attack rate (SAR) in families of affected students. We also compared the SAR of household contacts aged <18 years with those aged ⩾18 years. The protective role of oseltamivir was assessed by comparing the SAR of household contacts who had received oseltamivir prophylaxis with those who had not. Results were expressed in terms of odds ratio (OR) and 95% confidence interval (CI). We estimated the mean household serial interval (MSI) of pH1N1 virus by assessing the interval between onset dates of household index cases and secondary cases. Data analyses were performed using Microsoft Excel version 2003 and Epi Info version 3.5.1 (CDC, USA).
Of 511 students across six grades, we identified 65 cases (attack rate 12·7%). Grade 8 had the highest grade-specific attack rate (28·1%), followed by grade 9 (20·6%) and grade 10 (17·2%). None of the 153 school staff were affected. The first identified cases of the outbreak had symptom onset on 12 June 2009 and were in grade 8. The outbreak peaked on 14 June 2009 (Fig. 1). In order to stop disease transmission in the school, we advised the school to suspend classes for 2 weeks from 17 to 30 June 2009. The last case had onset of illness on 23 June 2009.
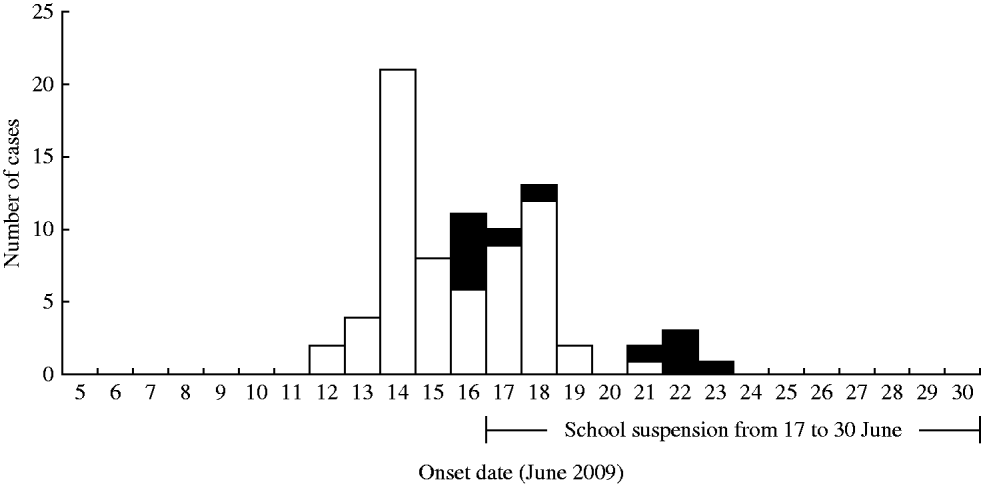
Fig. 1. Cases of pH1N1 infection in students (□) of school X (n=65), and infected household contacts (▪) of the affected students (n=12), by date of onset, Hong Kong, June 2009. The period of school suspension is indicated.
The median age of the affected students was 14 years (range 12–18 years). The male to female ratio was 1:1·2. The most common self-reported symptom was fever (62, 95·4%), followed by cough (53, 81·5%) and sore throat (37, 56·9%). Gastrointestinal symptoms occurred in 13·8% of the cases. All affected students were admitted to hospital for compulsory isolation. The median time interval from onset of illness to isolation was 3 days (range 0–7 days). There were no severe infections and all cases recovered without complications.
Of 205 household contacts identified, 12 persons from eight households were confirmed as having pH1N1 infection. All of them recovered uneventfully. The number of cases per household cluster (including the index case) ranged from two to four (median 2). The estimated SAR was 5·9% (12/205, 95% CI 2·7–9·1). The SAR in household contacts aged <18 years was 23·1% (9/39) compared to 1·8% (3/166) in contacts aged ⩾18 years (OR 16·3, 95% CI 3·7–81·3). The SAR was 0% in the 64 household contacts who had received oseltamivir prophylaxis compared to 8·5% (12/141) in those who had not (OR 0, 95% CI 0–0·9). The estimated MSI for pH1N1 virus was 2·8 days (range 2–4 days, 95% CI 2·1–3·4 days).
Reported school attack rates for confirmed pH1N1 infection in students have varied in the published literature. Two school outbreaks in New York City, USA, and Southeast England had attack rates of 4·5% and 4·7%, respectively, in students [Reference Lessler4, Reference Smith5], whereas an outbreak in a primary school in Birmingham, UK had an attack rate of 12·9% [6]. The attack rate in students in this outbreak was 12·7%, which is at the higher end of the reported range. During the outbreak, we sent all students who had fever or respiratory symptoms to hospital where respiratory specimens were collected for pH1N1 testing. This aggressive testing strategy may partly explain the apparently high attack rate in this outbreak.
The estimated SAR for pH1N1 virus in this outbreak was 5·9%. This is slightly lower than the 7–13% estimate reported by the WHO [7]. It is possible that previous exposure to other influenza viruses may have provided protective cross-immunity to some household contacts and thereby lowered the SAR. Our study suggests that the risk of secondary infection in household contacts aged <18 years was about 15 times higher than those aged ⩾18 years. This is consistent with results from a study conducted in Japan [Reference Odaira8], where the SAR in siblings was significantly higher than that in parents (16·4% vs. 2·5%, P<0·01).
Since all confirmed cases of pH1N1 infection were admitted to hospital for compulsory isolation during the study period, early separation of the index patient from household contacts might have lowered the SAR. However, we found no significant difference in SAR between households with index cases who were isolated >2 days after onset (SAR 4·9%) and households with index cases who were isolated ⩽2 days after onset (SAR 6·8%) (P=0·78). Moreover, other factors such as duration of contact with family members and personal hygiene standards of the index patient while at home may also affect SAR.
Our study also suggests that household contacts who had not received oseltamivir prophylaxis had a significantly higher attack rate than those who had received the prophylaxis. Similar findings were observed in the study conducted by Odaira et al. [Reference Odaira8] (OR 0·1, 95% CI 0–0·75). It appears that oseltamivir prophylaxis is effective in preventing secondary infection in household settings. However, this observation may be affected by other factors such as age of the household contacts and delay between index case onset and start of prophylaxis of the household contacts. Stratified analyses to assess how these factors affect the effectiveness of oseltamivir prophylaxis did not yield additional information as none of our secondary cases had received oseltamivir prophylaxis, which resulted in a crude OR and an adjusted OR of ‘0’.
The estimated MSI for pH1N1 virus in this study (2·8 days, 95% CI 2·1–3·4 days) was similar to the 2·6 days (95% credible interval 2·2–3·5 days) reported in a study conducted in the USA [Reference Cauchemez9]. The estimated MSI may be affected by factors such as early isolation of the index cases. However, further analysis revealed no significant difference in MSI between index patients with an onset to isolation interval of >2 days (2·4 days, 95% CI 1·3–3·5 days) and those with an onset to isolation interval of ⩽2 days (3 days, 95% CI 2·1–3·9 days) (P=0·3).
Considering the risk factors that may have contributed towards disease transmission in this outbreak, we found that about 22% (14/65) of the affected students continued to attend school for 1–4 days (median 1 day) despite being symptomatic. We also found mixing of students across classes in the same grade, a result of different student grouping for various subjects. Moreover, the school had extracurricular activities such as sports practice and arts performances, which may include students from different classes and grades. Students with pH1N1 infection who continued to attend school during the first few days of symptoms onset, coupled with mixing activities between students may have contributed to the widespread transmission of the virus in the school.
This study had several limitations. Owing to resources constraints we did not carry out active surveillance for all students and staff of the school. Therefore, we might have missed cases who did not seek medical attention resulting in an underestimation of the overall attack rate of the outbreak. Moreover, we did not conduct a serological survey in students, school staff and household contacts of confirmed cases, which would have enabled better assessment of the true attack rate in the school as well as SAR in the households.
The estimated SAR in this study should be interpreted with caution due to the small number of secondary cases. Moreover, some secondary cases might have been wrongly attributed to the household index as the infection could have been acquired from other sources, e.g. schoolmates. In our study, 9/12 secondary cases were either primary school or kindergarten students. Their onset dates ranged from 16 to 23 June 2009. Territory-wide school closure was implemented in Hong Kong for all primary schools and pre-primary institutions including kindergartens from 12 June 2009. Therefore, it was unlikely that these secondary cases acquired the infection from the school they attended.
As part of the containment strategy for the influenza pandemic, all confirmed cases of pH1N1 infection were admitted to hospital for compulsory isolation. Therefore, the 100% hospitalization rate for cases in this outbreak was unrelated to severity of illness.
Students of grades 8–10 had the highest grade-specific attack rates. However, we could not identify a single common activity or exposure in students from these three grades that could account for this observation.
In conclusion, this was an outbreak of pH1N1 infection affecting 65 students in a secondary school in Hong Kong. Secondary infection occurred in 12 household contacts of the affected students. The estimated SAR was 5·9%. Younger household contacts (aged <18 years) were significantly more likely to be infected than older contacts. Oseltamivir prophylaxis appeared to be effective in preventing secondary infection in household settings. The estimated MSI of pH1N1 virus was 2·8 days. The fact that students continued to attend school despite having symptoms and the considerable degree of mixing between students may have contributed towards the propagation of the outbreak.
ACKNOWLEDGEMENTS
We thank all staff of the Centre for Health Protection who contributed to the investigation and control of this outbreak. We also thank Dr Alain Moren and Dr Marta Valenciano for their comments on the manuscript.
DECLARATION OF INTEREST
None.



