Vitamin D is an essential micronutrient and plays an important role in bone metabolism( 1 , Reference Holick 2 ). The Institute of Medicine and the American Academy of Pediatrics suggest that serum 25-hydroxyvitamin D (25(OH)D) concentration of 50 nmol/l will meet the needs of 97·5 % of the population for optimal bone-related health outcomes( 3 , Reference Wagner and Greer 4 ).
There is emerging evidence that low serum 25(OH)D levels may also be associated with a number of chronic health problems( Reference Bischoff-Ferrari, Giovannucci and Willett 5 – Reference Bischoff-Ferrari, Willett and Wong 8 ). Observational epidemiological studies have suggested that low levels of vitamin D may play a role in fractures( Reference Dawson-Hughes 9 , Reference Clark, Tobias and Ness 10 ), asthma( Reference Litonjua and Weiss 11 , Reference Masoli, Fabian and Holt 12 ), respiratory infections( Reference Ginde, Mansbach and Camargo 13 ) and obesity( Reference Reis, von Muhlen and Miller 14 ) in children. Identifying subgroups of children who are at risk of having low 25(OH)D is important, especially given the possibly long duration of exposure to low 25(OH)D beginning in childhood( 1 , Reference Holick 2 ).
A number of determinants have been identified that affect 25(OH)D levels in children including skin pigmentation, breast-feeding without vitamin D supplementation, low intake of cow's milk (in Canada and the USA, cow's milk is fortified with approximately 2·5 μg (100 IU) of vitamin D per 250 ml), higher latitude and higher adiposity( Reference Greer 15 – 29 ). It has been suggested that immigration may also play a role in vitamin D status. Observational epidemiological data have suggested that non-Western adults immigrating to a Western country (Europe, North America, Australia or New Zealand) are at increased risk of having low 25(OH)D( Reference Skull, Ngeow and Biggs 30 – Reference Andersen, Molgaard and Skovgaard 34 ). Further, children under 1 year of age from non-Western immigrant families living in a Western country appear to be at risk of developing vitamin D-deficiency rickets( Reference Ward, Gaboury and Ladhani 35 – Reference Pillow, Forrest and Rodda 37 ). It is not known whether there is a relationship between non-Western immigration and 25(OH)D during early childhood (i.e. in children older than 1 year of age) and whether dietary, environmental or biological determinants of 25(OH)D might explain this effect.
The primary objective of the present study was to determine whether children older than 1 year of age from non-Western immigrant families have lower serum 25(OH)D levels than children from Western-born families. Our secondary objective was to evaluate whether known dietary, environmental or biological determinants of 25(OH)D influence this relationship.
Methods
The present study was a cross-sectional observational study of healthy children aged 1–6 years.
Participants
Children were recruited between December 2008 and July 2011 during a routine well-child doctor's visit at seven paediatric and family medicine group practices participating in TARGet Kids!, and represented a diverse sample of children in inner-city Toronto (latitude 43·4°N), the most culturally diverse city in Canada. Details of subject recruitment have been published elsewhere( Reference Morinis, Maguire and Khovratovich 38 , 39 ). The TARGet Kids! practice-based research network was designed to collect data relevant to nutritional factors and dietary patterns in healthy infants and children. It was developed as a partnership between researchers at the Paediatric Outcomes Research Team at the Sick Kids Research Institute of The Hospital for Sick Children, the Applied Health Research Centre at the Li Ka Shing Knowledge Research Institute of St. Michael's Hospital, and primary-care providers in the Section on Community Paediatrics in the Department of Paediatrics and the Department of Family and Community Medicine at the University of Toronto. Exclusion criteria included any chronic illness (except for asthma), severe developmental delay, non-verbal English and medications known to affect vitamin D metabolism (i.e. anti-seizure medications).
Measurements
Survey data were collected through a parent-completed standardized data collection form adapted from the Canadian Community Health Survey( 40 ). Trained research assistants embedded in the practices obtained physical measurements and venous sampling occurred on site at the primary-care clinic by a trained phlebotomist. Blood samples were sent daily to the Mount Sinai Services Laboratory in Toronto (www.mountsinaservices.ca).
Serum 25(OH)D was measured using a competitive two-step chemiluminescence assay (Diasorin LIAISON®)( 41 ). This assay was regularly calibrated according to the internationally recognized Vitamin D External Quality Assessment Scheme( Reference Carter, Carter and Jones 42 ). Extensive testing and validation of this assay have been performed and demonstrated an intra-assay imprecision of 7·2 % at a concentration of 213 nmol/l and an inter-assay imprecision of 4·9 % at 32 nmol/l, 8·9 % at 77 nmol/l and 17·4 % at 213 nmol/l, values which are well within acceptable limits for biochemical measurements( Reference Maunsell, Wright and Rainbow 43 , Reference Singh, Taylor and Reddy 44 ).
Our primary exposure variable was non-Western immigration determined by the parent(s) and child's country of birth. Non-Western immigration was defined as a child born outside Europe, North America, Australia or New Zealand or a child who has a parent (one or both) who emigrated from a non-Western country( Reference Schenk, Ellert and Neuhauser 45 ). Thus first- and second-generation non-Western immigrant children were considered non-Western immigrants for the present analysis because dietary factors affecting young children likely reflect cultural patterns of their parents( Reference Nusche, Wurzburg and Naughton 46 – Reference Schwartz, Montgomery and Briones 51 ). Immigration was measured by two open-ended questions: ‘Where were your child's biological parents born?’ and ‘Where was your child born?’
Our primary outcome was serum total 25(OH)D (continuous outcome) and our secondary outcome was 25(OH)D < 50 nmol/l (binary outcome), based on the Institute of Medicine's reference cut-off point( 3 ).
Clinically relevant covariates that we hypothesized might influence the relationship between non-Western immigration and 25(OH)D included ethnicity, sex, age, skin pigmentation, BMI, season, current vitamin D supplementation, cow's milk intake and outdoor play. Ethnicity was captured by the open-ended ethnicity question ‘What were the ethnic or cultural origins of your child's ancestors (an ancestor is usually more distant than a grandparent)?’ Two co-authors, J.A.O. and S.C., independently converted responses into the following five geographically based ethnicity categories: (i) East & South-east Asian; (ii) South-west Asian; (iii) African & Caribbean; (iv) mixed Western; and (v) mixed Western/non-Western( 52 ). Mixed Western included children born in families from Western countries (e.g. Western and Eastern Europe) and mixed Western/non-Western included children from mixed ethnic families from both non-Western countries (e.g. East Asian and Latin American) or families from Western and non-Western countries (e.g. South Asian and Western Europe). Differences in categorization between reviewers were identified less than 5 % of the time and subsequently resolved by consensus in each instance. The overall effect of ethnicity was tested using Western as the reference of the other four geographically based ethnic categories, identified above.
Each child's weight was measured using a precision digital scale (±0·025 %; Seca, Hamburg, Germany) and standing height was measured using a stadiometer (Seca). BMI was calculated as weight in kilograms divided by the square of height in metres( Reference Pietrobelli, Faith and Allison 53 , Reference Mei, Grummer-Strawn and Pietrobelli 54 ). BMI Z-scores were calculated using WHO growth standards( 55 ). Skin pigmentation was measured by trained research assistants using the Fitzpatrick scale, which is a skin pigmentation classification system widely used in dermatological research( Reference Fitzpatrick 56 , Reference Quevedo, Fitzpatrick and Pathak 57 ). Cow's milk consumption was measured from parental report based on response to the following question: ‘How many 250 ml cups of cow's milk does your child drink in a typical day?’ All commercially available cow's milk in Canada is fortified with 2·5 μg (100 IU) of vitamin D per 250 ml cup( Reference Roth, Martz and Yeo 58 , 59 ). Daily vitamin D supplementation was defined as currently taking a daily multivitamin and/or vitamin D supplement. In Canada, all over-the-counter multivitamins contain vitamin D and standard dosing of children's vitamin D-containing supplements is 10 μg (400 IU) per dose( 60 ). Outdoor play was defined as hours per week spent outside playing, which was used as a proxy for sun exposure.
Statistical analyses
Descriptive statistics were performed for the primary exposure, outcomes and covariates. For our primary analysis, univariate linear regression was used to determine the unadjusted association between our primary exposure (non-Western immigration) and our primary outcome (serum 25(OH)D as a continuous outcome) and univariate logistic regression was used to determine the unadjusted association between our primary exposure (non-Western immigration) and our secondary outcome (25(OH)D < 50 nmol/l as a binary outcome). For our secondary analysis, a multiple linear regression model was developed using our primary outcome, serum 25(OH)D, with adjustment for pre-specified, clinically relevant covariates (described above) to explore factors which might influence a relationship between non-Western immigration and 25(OH)D. All covariates were felt to be clinically important and were included in the final model regardless of P value.
To explore whether vitamin D supplementation and skin pigmentation may have different effects on 25(OH)D in non-Western immigrant children relative to Western-born children, two biologically plausible interactions were considered: (i) immigration and skin pigmentation; and (ii) immigration and vitamin D supplementation. To achieve a balance between over-fitting and interpretation and limit biases that can result from standard variable selection approaches, these interactions were tested together using a likelihood ratio test. If the P value for inclusion of the interactions was large (i.e. greater than 0·30), these interactions were considered to be unlikely and were not included in the final models.
Multicollinearity was assessed using the variance inflation factor, a measure of the degree that a regression coefficient is inflated when other independent variables contain similar information( Reference Cody and Smith 61 ). As the model did not contain large variance inflation factors (values not exceeding 5) multicollinearity was unlikely to be a problem, so each of the hypothesized covariates (including ethnicity and immigration) were considered independent variables( Reference Chui, Tran and Maheux 62 ).
Data were analysed using the statistical software package SAS 9·2 for Windows. The study was approved by the Research Ethics Board of St. Michael's Hospital and The Hospital for Sick Children, and parents of all participating children consented to participation in the study.
Results
Consent was obtained from parents of 3696 children; 1540 had complete survey, anthropometric and laboratory data and were included in the analysis (see Fig. 1). Children included and not included in the analysis appeared similar (see Table 1). The median age of included children was 36 months, 51 % were male, 86 % had ‘light’ skin pigmentation (Fitzpatrick scale I, II or III), 55 % took vitamin D supplements and mean cow's milk intake was 1·8 cups/d. Children from non-Western immigrant families made up 27 % of the population (see Table 2). Of non-Western immigrant families, 4 % of children and 96 % of parents were born outside Canada, in a non-Western country. Median serum 25(OH)D was 83 nmol/l. Eighty-one children (5 %) had 25(OH)D levels below 50 nmol/l (thirty-one (3 %) children from Western families and fifty (12 %) children from non-Western immigrant families).
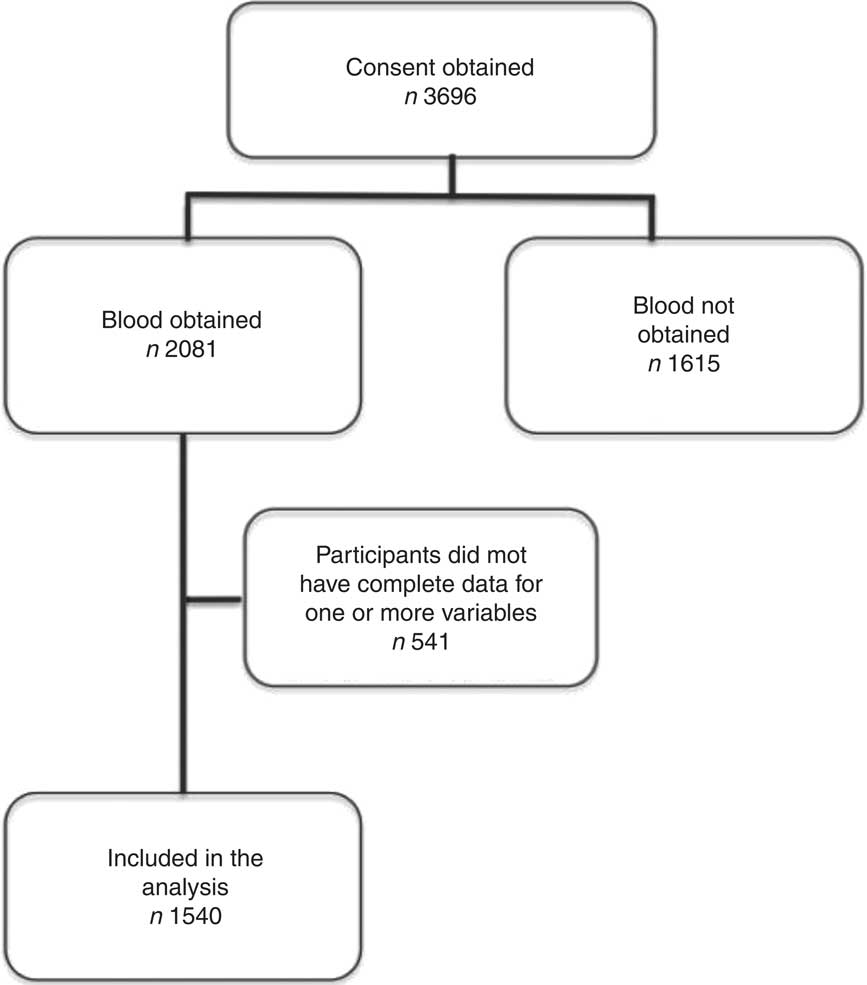
Fig. 1 Patient recruitment and enrolment flow chart
Table 1 Population description for children included and not included in the analysis (children aged 1–6 years participating in TARGet Kids!, Toronto, Canada, December 2008 to July 2011)

25(OH)D, 25-hydroxvitamin D.
*2176 children were not included in the analysis (blood not obtained for 1615 and 1540 did not have complete survey or anthropometric data). The table describes 1979 children; 197 were removed because they were outliers (fifteen children had BMI Z-score > 4, 181 had age <11·5 months or >84 months, and one had 25(OH)D of 352 nmol/l).
†Visible minorities in Toronto according to the 2006 census were 12·0 % South Asian, 2·6 % Arab or West Asian (totalling 14·6 % South-west Asian); 11·4 % Chinese, 4·1 % Filipino, 1·4 % Korean, 1·5 % South-east Asian, 0·5 % Japanese (totalling 18·9 % East Asian & South-east Asian); 8·4 % Black (totalling 8·4 % African & Caribbean); and multiple visible minority 1·3 %( 72 ).
‡Median annual household income in Toronto in 2010 was $CAN 68 110( 73 ).
Table 2 Population description for children from Western-born families and from non-Western immigrant families (children aged 1–6 years participating in TARGet Kids!, Toronto, Canada, December 2008 to July 2011)

25(OH)D, 25-hydroxvitamin D.
For our primary analysis, univariable linear regression revealed that non-Western immigrant children had lower mean serum 25(OH)D concentrations than children from Western-born families (85 v. 89 nmol/l, respectively) with a difference of 4 (95 % CI 1·3, 8·0) nmol/l (P = 0·006). Univariable logistic regression revealed increased odds of 25(OH)D levels less than 50 nmol/l in non-Western immigrant children (OR = 1·9; 95 % CI 1·3, 2·9).
For our secondary analysis, multiple linear regression adjusted for clinically relevant covariates resulted in a reduction of the observed mean serum 25(OH)D difference between non-Western immigrant children and children from Western-born families to 0·04 (95 % CI −4·8, 4·8) nmol/l, which was no longer statistically significant (P = 0·99; see Table 3).
Table 3 Adjusted linear regression model for the association between immigration status and serum 25(OH)D (among children aged 1–6 years participating in TARGet Kids!, Toronto, Canada, December 2008 to July 2011)

25(OH)D, 25-hydroxvitamin D.
*Indicates those variables that are independently associated with serum 25(OH)D (P < 0·05).
†The effect of ethnicity in the model was tested using mixed Western as the reference for the other four geographically based ethnic categories. The reported P value represents the statistical significance of a likelihood ratio test for all ethnicities tested in the model together relative to the reference.
Covariates which appeared to attenuate the relationship between non-Western immigration and 25(OH)D included volume of cow's milk intake (P < 0·0001), vitamin D supplementation (P = <0·0001), season (P = 0·008) and age (P = 0·04; see Table 3). Cow's milk intake, vitamin D supplementation, season and age were all associated with non-Western immigration and had an effect on 25(OH)D. However, only vitamin D supplementation changed the parameter estimate for non-Western immigration by more than 10 %, suggesting it was the strongest explanatory factor. We did not find that other variables, including ethnicity, skin pigmentation and outdoor play, were modifiers of the observed 25(OH)D difference (see Table 3).
Interactions between non-Western immigration and vitamin D supplementation and non-Western immigration and skin pigmentation were tested together which revealed P = 0·9, making these interactions unlikely. These interactions were not included in the final model.
Discussion
Immigration is a defining component of urban North America( Reference Chui, Tran and Maheux 63 , Reference Grieco, Acosta and de la Cruz 64 ). We have identified an association between non-Western immigration and lower 25(OH)D in early childhood. While the median 25(OH)D concentration was 83 nmol/l, well above the American Academy of Pediatrics’ cut-off point of 50 nmol/l, non-Western immigrant children had nearly a twofold increased odds of 25(OH)D < 50 nmol/l when compared with children from Western-born families.
When biologically important covariates related to vitamin D intake and synthesis were included in our adjusted model, the observed 25(OH)D mean difference between immigration groups could largely be explained by known vitamin D determinants, with current vitamin D supplementation having the strongest effect. Cow's milk intake, season and age were significant covariates in the adjusted linear regression model but did not change the parameter estimate for non-Western immigration by more than 10 %, suggesting they were weaker explanatory factors.
To our knowledge, the present study is unique in documenting an association between 25(OH)D status and non-Western immigration in early childhood (children aged 1–6 years). Understanding non-Western immigration as an exposure is important due to the high frequency of non-Western immigration in much of urban North America( Reference Chui, Tran and Maheux 63 ). Our finding that vitamin D supplementation appears to be the strongest explanatory factor of the observed difference in 25(OH)D suggests that vitamin D supplementation may be an excellent target for interventions to increase 25(OH)D among non-Western immigrant children.
Previous studies have identified a number of factors that affect 25(OH)D in children including factors related to cutaneous production of vitamin D such as skin pigmentation (melanin pigment decreases cutaneous synthesis)( Reference Greer 15 – Reference Carpenter, Herreros and Zhang 17 ), ethnicity( Reference Hintzpeter, Scheidt-Nave and Müller 65 , Reference McGillivray, Skull and Davie 66 ) and outdoor time( Reference Greer 15 – Reference Carpenter, Herreros and Zhang 17 , Reference Tandon, Zhou and Christakis 67 , Reference Maguire, Birken and O'Connor 68 ). We did not find that these factors were modifiers of the relationship between non-Western immigration and 25(OH)D. This could be a consequence of sun avoidance of young children or the relatively low frequency of ‘dark’ skin pigmentation in this population. If skin exposure to the sun were minimal, cutaneous production of 25(OH)D would also be expected to be minimal regardless of skin pigmentation, ethnicity or outdoor playtime.
Strengths of our study were the relatively large sample size with detailed clinical and laboratory data which allowed us to adjust for the many factors known to impact 25(OH)D concentrations in children. Further, our urban population included an ethnically diverse sample from one of the most multicultural cities in the world.
Limitations of the study include its cross-sectional design, from which causality cannot be inferred. Although the median 25(OH)D concentration was relatively high in our population and the majority of children had 25(OH)D levels above the American Academy of Pediatrics’ cut-off point of 50 nmol/l, other Canadian-based studies including the national Canadian Health Measures Survey have found similar 25(OH)D levels in this age group( Reference El Hayek, Pham and Finch 69 – Reference Langlois, Greene-Finestone and Little 71 ). There was a low representation of certain ethnic groups in the present study compared with visible minority groups in Toronto; however, this can be partially explained by the higher frequency of mixed ethnicities in our study( 72 ). Residual confounding from unknown and unmeasured covariates is also a possibility, although such effects are likely to be small given that the adjusted 25(OH)D difference was small. Finally, a language barrier could have precluded some immigrant families from participating in the study. However, only 0·4 % of eligible children were actually excluded because of a language barrier yet almost a third of our population were non-Western immigrant families.
Conclusion
Children older than 1 year of age from non-Western immigrant families may be at increased risk of lower 25(OH)D. Vitamin D supplementation appeared to be the strongest explanatory factor of the observed difference in 25(OH)D, suggesting that targeted interventions to improve vitamin D supplementation among immigrant children beyond the first year of life may be successful at increasing the 25(OH)D status of non-Western immigrant children. Non-modifiable factors such as ethnicity and skin pigmentation did not appear to explain the observed difference.
Acknowledgements
Sources of funding: This work was supported by an unrestricted master's award from the Canadian Institutes of Health Research (CIHR), priority announcement Nutrition and Dietetic Research (SHOPP) in partnership with the Canadian Foundation for Dietetic Research. Overall support for the TARGet Kids! programme was provided by the CIHR Institute of Human Development, Child and Youth Health (IHDCYH) and the Institute of Nutrition Metabolism and Diabetes (INMD), as well as the St. Michael's Hospital Foundation. The Paediatric Outcomes Research Team (PORT) is supported by a grant from The Hospital for Sick Children Foundation. The funding agencies had no role in the design, collection, analyses or interpretation of the study results. Conflict of interest: The authors have no financial relationships relevant to this article and no conflicts of interest to disclose. Authors’ contributions: J.A.O. and J.L.M. designed the research study. P.B.D., P.C.P. and C.S.B. helped to refine the study design. J.A.O., J.L.M. and K.E.T. analysed the data. M.K., S.C. and J.D. coordinated data collection. All authors contributed to the interpretation of results. J.A.O. and J.L.M. drafted the manuscript. All authors read and approved the final manuscript. Acknowledgements: The authors thank the practitioners, paediatric and family medicine practices and families who are currently involved in the TARGet Kids! research network.
TARGet Kids! Collaboration Site Investigators: Tony Barozzino, Gary Bloch, Ashna Bowry, Douglas Campbell, Sohail Cheema, Brian Chisamore, Karoon Danayan, Anh Do, Michael Evans, Mark Feldman, Sloane Freeman, Moshe Ipp, Sheila Jacobson, Tara Kiran, Holly Knowles, Eddy Lau, Fok-Han Leung, Julia Morinis, Sharon Naymark, Patricia Neelands, Michael Peer, Marty Perlmutar, Navindra Persaud, Michelle Porepa, Noor Ramji, Alana Rosenthal, Janet Saunderson, Michael Sgro, Susan Shepherd, Carolyn Taylor, Sheila Wijayasinghe, Ethel Ying and Elizabeth Young. Steering Committee: Tony Barozzino, Brian Chisamore, Mark Feldman and Moshe Ipp. Research Team (Managers/Coordinators/Research Assistants): Azar Azad, Tonya D'Amour, Sarah Carsley, Julie DeGroot, Kanthi Kavikondala, Marina Khovratovich, Tarandeep Malhi, Magda Melo, Subitha Rajakumaran, Juela Sejdo and Laurie Thompson. Applied Health Research Centre: Muhammad Mamdani, Andreas Laupacis, David Klein, Gerald Lebovic, Kevin Thorpe, Magda Melo, Kim Phu, Judith Hall and Rino La Grassa, Bryan Boodhoo, Nike Onabajo, Karen Pope.






