Folate is a water-soluble B vitamin that is naturally present in some foods (polyglutamate form), especially fruits, vegetables (particularly dark green leafy vegetables), legumes, dairy products, poultry and meat and that is also available as a dietary supplement (monoglutamate form, folic acid)( Reference Ebara 1 ). Folate has a crucial role on one-C metabolism as one-C acceptor and donor and is also well known to act as a coenzyme, which may be involved in pyrimidine and purine synthesis and various methylation reactions( Reference Stover 2 , Reference Bailey and Caudil 3 ).
Low folate status has been associated with an increased risk of chronic diseases such as CVD( Reference Wang, Zhou and Nie 4 ), cancer( Reference Chen, Xu and Liu 5 ) and cognitive impairment( Reference Cooper, Sommerlad and Lyketsos 6 ). Hyperhomocysteinemia, which is positively related to folate deficiency, has been also associated with increased risk of CVD and cerebrovascular diseases in epidemiological studies( Reference Blom and Smulders 7 , Reference McCully 8 ), although a meta-analysis of B vitamin supplementation including folate showed no reduction of the risk of vascular diseases( Reference Bailey, da Silva and West 9 ). Diabetes is a well-known risk factor for the development of CVD and cerebrovascular diseases( Reference Grundy, Benjamin and Burke 10 ) and is affected by environmental factors as well as genetic factors( Reference Kato 11 ). Previous meta-analyses showed the epidemiological evidence for associations of type 2 diabetes (T2D) with education level( Reference Agardh, Allebeck and Hallqvist 12 ), smoking status( Reference Willi, Bodenmann and Ghali 13 ), drinking status( Reference Li, Yu and Zhou 14 ), regular exercise( Reference Jeon, Lokken and Hu 15 ), BMI/waist circumference (WC)( Reference Vazquez, Duval and Jacobs 16 ), and some dietary components such as processed red meat, sugar-sweetened beverage, cereal fibre and whole grains, etc.( Reference Ley, Hamdy and Mohan 17 ). Among dietary factors, epidemiological evidence regarding the relationship between dietary folate intake and T2D is lacking. However, in a meta-analysis( Reference Huang, Ren and Huang 18 ), the negative association between a methylene-tetrahydrofolate reductase (MTHFR) variant (677C>T) and the development of T2D suggested the beneficial effect of folate on T2D.
Until recently, there has not been a prospective study evaluating whether higher dietary folate intake is associated with a lesser chance of developing diabetes. Therefore, we examined the prospective association between folate intake and T2D risk among men and women aged 40 years or older in a community-based cohort in South Korea.
Methods
Study design and population
This prospective cohort study was the ongoing Multi-Rural Communities Cohort, part of the Korean Genome and Epidemiology Study (KoGES). Participants aged 40 years or over (n 9692) were recruited from three rural areas in the Republic of Korea – Yangpyeong, Namwon and Goryeong – between January 2005 and December 2009 for an investigation of CVD risk factors. Follow-up surveys for re-examination similar to the baseline examination were conducted every 2–4 years (average 2·85 years) after the baseline survey. Participants returned for a second examination between 2007 and 2013 (mean of follow-up duration, 2·5 (sd 1·0) years) and for a third examination between 2010 and 2013 (mean of follow-up duration, 3·2 (sd 1·0) years). Of total participants (n 9692), 7640 participants (78·0 %) for the second examination and 4040 participants (41·7 %) for the third examination completed. Average follow-up years were 4·06 (sd 1·96) years. Participants who had a history of heart disease, stroke and/or cancer (n 1116); who had been treated with anti-diabetic drugs or insulin; or whose fasting blood glucose (FBG) level at baseline was 7·0 mmol/l (126 mg/dl) or higher (n 976) were excluded. In addition, those with implausible dietary intakes (<2092 or >16 736 kJ/d (<500 or >4000 kcal/d) or >10 missing food items; n 77), with insufficient blood specimens, or with missing data for important covariates needed to identify the association between diabetes and folate intake (n 190) were removed. Thus, 7333 participants (2693 men and 4640 women) were included in the final analysis. This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Boards of Hanyang University, Chonnam National University and Keimyeong University.
Dietary assessment
To assess dietary nutrient intake, we used a previously validated semi-quantitative FFQ composed of 106 food items( Reference Ahn, Kwon and Shim 19 ). Trained interviewers asked participants to report how often they had consumed each item over the past year, using nine frequency categories ranging from ‘never or rarely’ to ‘3 times/d,’ as well as three standardised portion sizes. In the case of limited seasonal food items, additional data were collected on how long these items were eaten (for 3, 6, 9 or 12 months of the year). Dietary nutrient intakes, including folate intake, were calculated by multiplying the reported intake frequency of each food by the amount of nutrients in a serving size of that food based on nutrient database of CAN-PRO 4.0 (Computer Aided Nutritional analysis program) of the Korean Nutrition Society( Reference Society 20 ). For seasonal foods, seasonal intake period was additionally considered to calculate nutrient intake. To isolate variation in nutrient intake due only to the nutrient composition of the diet from extraneous variation by the correlation with total energy intake( Reference Willett 21 ), total energy-adjusted-nutrient intakes were estimated by residual method, which uses the regression model with total energy intake as the independent variable and nutrient intake as the dependent variable. Nutrients intake from supplements could not be calculated, because there was no publicly available well-established national database and because most participants did not correctly recall the brand name.
Assessment of non-dietary factors
Data were collected from questionnaires and examinations according to standard protocols and procedures. Study participants were asked about demographic information including age, education level, smoking status, alcohol consumption, physical activity, medical history, menopause status and supplement use by trained interviewers. For anthropometric assessment, height and weight were measured to the nearest 0·1 cm and 0·1 kg, respectively, whereas subjects were dressed in light clothing. BMI was calculated as the ratio of weight (kg) to squared height (m2). WC was measured at the midpoint between the lowest rib margin and the iliac crest. Systolic and diastolic blood pressure were measured after >5 min of rest in the seated position, by means of a standard sphygmomanometer and a cuff on the right arm. Blood pressure was measured at least twice, at 5-min intervals, and the last two measurements were averaged for the analysis. From blood samples collected after an 8-h fast, FBG levels was analysed on an ADVIA1650 Automatic Analyzer (Siemens).
Ascertainment of type 2 diabetes
Participants were asked to report a new diagnosis of diabetes at every visit. Those reporting physician-diagnosed diabetes were asked whether they were being treated with oral anti-diabetic medication and/or insulin. Based on the criteria of the American Diabetes Association( 22 ), subjects were confirmed to have diabetes if they exhibited at least one of the following: (1) elevated FBG≥126 mg/dl (7·0 mmol/l) or (2) treatment with hypoglycaemic medication (insulin or oral drugs).
Statistical analysis
We calculated the person time for each participant from the baseline examination date until T2D diagnosis, death from any cause or censoring. If participants were lost to follow-up, their follow-up times were assigned as half the median of the person years of participants who properly completed follow-up( Reference MacMahon and Pugh 23 ). We analysed the association of diabetes with two different measures of folate intake, baseline folate consumption and average folate consumption. The latter might reduce measurement error and/or more effectively represent long-term dietary patterns, because measured changes in diets of individuals over time are a mix of true variation and measurement error( Reference Ley, Hamdy and Mohan 17 ). The average dietary consumption of folate was calculated by averaging the intake at baseline and at repeated examination just before the censoring time or endpoint of each subject. The general characteristics of the participants were described as means and standard deviations for continuous variables and as numbers and percentages for categorical variables.
Participants were divided into tertiles of folate intake. The low intake group, moderate intake group and high intake group were presented as the first tertile (T1), the second tertile (T2) and the third tertile (T3), respectively. Potential confounding factors were selected based on previously reported association with T2D in the meta-analysis using prospective cohort results (education level( Reference Agardh, Allebeck and Hallqvist 12 ), smoking status( Reference Willi, Bodenmann and Ghali 13 ), drinking status( Reference Li, Yu and Zhou 14 ), regular exercise( Reference Jeon, Lokken and Hu 15 ) and BMI/WC( Reference Vazquez, Duval and Jacobs 16 )). Likewise, for dietary factors, nutrients and foods previously reporting the significant association with T2D in the meta-analysis of prospective cohort studies (haeme-Fe, glycaemic index/glycaemic load, Mg, cereal fibre, processed meat, unprocessed red meat, white rice, vegetables, dairy product, sweetened beverages and coffee consumption)( Reference Ley, Hamdy and Mohan 17 ) and randomised clinical trial (total energy intake( Reference Anderson, Kendall and Jenkins 24 )) were first considered as potential confounders. However, haeme-Fe and cereal fibre could not be calculated because of lacking of database. Fe from animal foods and fibre from cereals and grains were used. Among those factors, factors showing statistically significant difference (Tukey’s multiple comparison) or trend according to the tertiles of folate intake were selected using the general linear model after controlling for age. For the test of linear trends, the median folate intake values of the tertiles were treated as continuous variables, and the level of significance was set at 0·05. To reduce collinearity among covariates, the following steps were taken; First, age-adjusted bivariate correlations were evaluated among covariates. Then, if covariates were highly correlated with dietary folate intake at Pearson’s r≥0·6, they were not included in the final analysis and if there was high correlation between two covariates, we decided whether to select which covariate as a potential confounder. Next, among nutrients and foods that may reflect the same aspects of diet such as carbohydrate, glycaemic index, glycaemic load and white rice, only one variable was selected. Because subjects may have modified their diets after recognising their own relatively high FBG level and the follow-up period in the present study was relatively short, we adjusted for baseline FBG. In addition, the modified Alternative Healthy Eating Index( Reference McCullough, Feskanich and Stampfer 25 ) using Korean Dietary Reference Intakes (KAHEI) was also included to reduce confounding effect due to the relation to a healthy diet of dietary folate intake.
To estimate the incidence rate ratios (IRR) and 95 % CI according to tertile of dietary folate intake, we used a modified Poisson regression model with a robust error estimator( Reference Zou 26 , Reference Callas, Pastides and Hosmer 27 ). The age-adjusted model and the multivariable-adjusted models were employed. We could not isolate prediabetes due to no oral glucose tolerance test (OGTT) data in the present study, but it could be confirmed that there were just a few prediabetes by OGTT among participants with FBG<100 mg/dl (<5·6 mmol/l, 3·13 % in another community cohort, a part of KoGES). Therefore, the association between dietary folate intake and T2D were separately analysed in normal fasting glucose (NFG) group with <100 mg/dl FBG value (5·6 mmol/l) and in impaired fasting glucose (IFG) group which was defined as ≥100 and <126 mg/dl of FBG value (5·6–6·9 mmol/l) by the American Diabetes Association( 22 ). The interaction of dietary folate intake with FBG groups and sex were evaluated in the modified Poisson regression model. Taking nutrient supplements is a health-related behaviour and also provides folic acid( Reference Lyle, Mares-Perlman and Klein 28 ). Therefore, we additionally analysed the results among supplement non-users after excluding users of only multinutrients and after additionally excluding users of vitamin supplements such as vitamin A, vitamin C, β-carotene, vitamin B complex and vitamin E.
For sensitivity analysis, the analyses using two different person times assignment to loss to follow-up were conducted( Reference MacMahon and Pugh 23 ). The first assignment was based on the assumption that all persons were lost immediately after the last date they were known to be present, and the second was based on the assumption that they were all lost immediately before the first date on which they were known to be absent. At the second assignment, we assigned the median person time between visits. It assumed that the correct rate lied somewhere between two rates. Furthermore, a possible difference in the whole dietary patterns between the lowest and highest tertile of dietary folate was investigated using radar charts( Reference Würtz, Hansen and Tjønneland 29 , Reference Ryu and Hong 30 ). To illustrate radar chart, first, twenty-two food groups (fruit, mushroom, seaweed, poultry, unprocessed red meat, processed red meat, fish/seafood/shell, egg, dairy products, pulses/soya, nut/seed, coffee, snack/sugar, sugar-sweetened beverage, alcohol consumption, whole grain, refined grain, noodle/bred, potato/starch, green leafy vegetable, other vegetables and salted vegetable) were calculated using foods in recipe of the FFQ. The median intake (g/d) of each food group for participants in T1 (or T3) of folate intake was compared with their median intake values for the entire population to obtain the relative percentage of food or beverage intake. Radar chart was illustrated using those relative percentages for the baseline and the average folate intake among men and women. The intake of food groups (g/d) was energy-adjusted value using the residual method( Reference Willett 21 ). SAS software (version 9.4; SAS Institute Inc.) and R-software (version 3.4.0, http://www.r-project.org) were used for all statistical analyses.
Results
The study participants included 2693 men (36·7 %) and 4640 women (63·3 %). The average age was 62·0 (sd 9·7) years among men and 60·7 (sd 9·9) years among women. Men were more likely to be current drinkers (66·7 v. 30·2 %) and current smokers (35·8 v. 3·6 %) and have higher education (29·6 v. 14·3 %) than women. Table 1 displays the age-adjusted characteristics of participants as potential confounders by sex. The median value of baseline folate intakes among men and women were 369·7 and 333·7 μg/d, respectively. Compared with T1, the higher proportion of participants in T3 had higher education levels, performed regular exercise, IFG and consumed multinutrients and/or vitamins in both men and women, whereas a lower proportion in T3 was current smoker. Participants in T3 had also higher mean WC and FBG in both men and women. Among dietary factors, the higher average consumptions in T3 compared with T1 were found in protein, fat, Fe from animal sources, Mg, total vegetables, dairy products and KAHEI in both men and women, whereas the lower consumptions in T3 were found in energy, carbohydrate, glycaemic index, glycaemic load, white rice, sweet beverages and coffee consumption. We found that women in T3 were younger and had higher alcohol consumption and lower fibre from cereals and grains and men in T3 had lower processed meat. There were similar trends in these characteristics according to tertile of average dietary folate intake (online Supplementary Table S1).
Table 1 Age-adjusted characteristics of the study participants according to tertiles (T) of folate intake at baseline survey (Mean values with their standard errors; numbers and percentages)
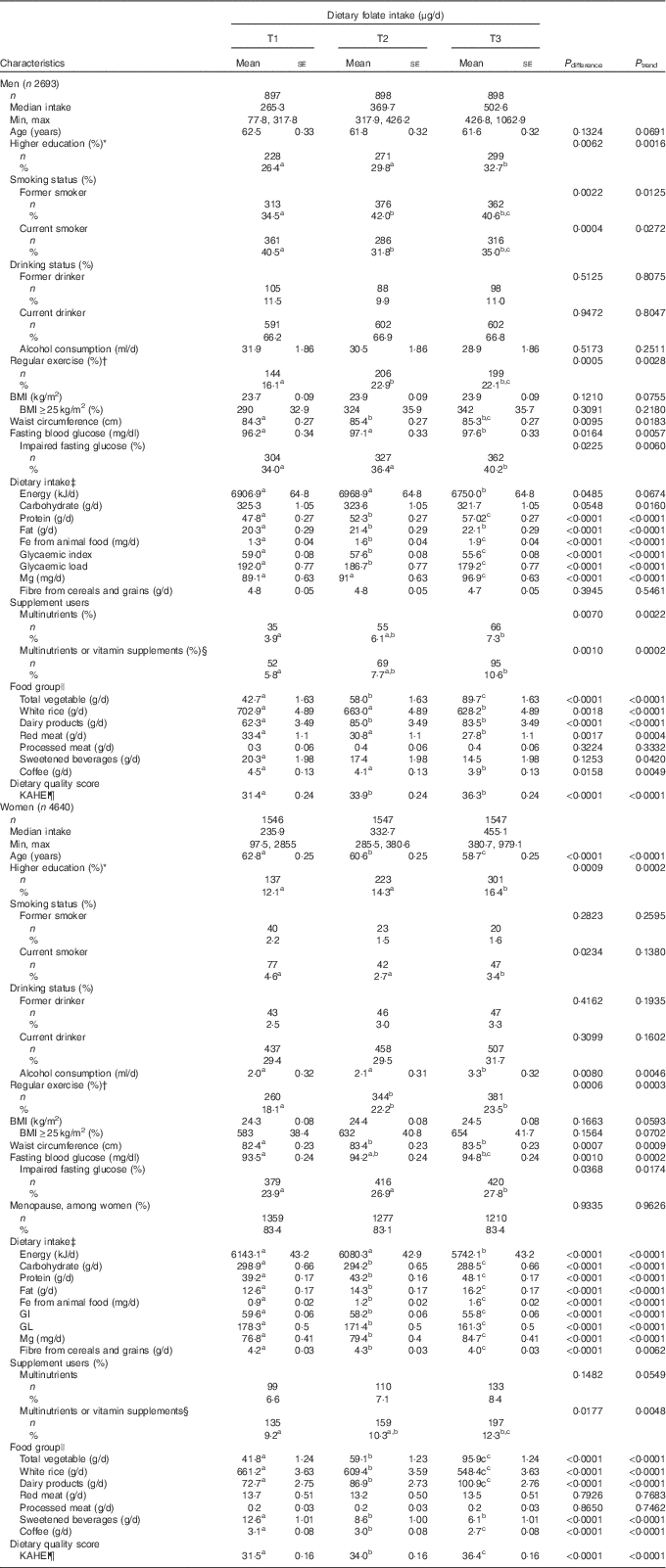
KAHEI, Korean Alternative Healthy Eating Index.
a,b,c Values within a row with unlike superscript letters were significantly different among the groups by Tukey’s multiple-comparison test.
* High school graduation or longer (≥12 years of education).
† ≥3 times/week and 30 min/session.
‡ All nutrients were total energy-adjusted values.
§ Vitamin supplements included supplements of vitamin A, vitamin C, β-carotene, vitamin B complexes and vitamin E.
|| Age- and total energy-adjusted means for food groups using general linear model.
¶ KAHEI was the modified alternative healthy eating index based on Korean Dietary Reference Intakes.
Table 2 displays the IRR and 95 % CI according to dietary folate intake. For 29 745 (sd 1·96) person years of follow-up, 319 participants developed de novo diabetes. Baseline folate intake was inversely associated with T2D risk in T3 compared with T1 after adjustment for all multiple confounding factors (IRR=0·57; 95 % CI 0·38, 0·87, P for trend=0·0085) among women. This inverse association was similar when the average folate consumption was used (IRR=0·64; 95 % CI 0·43, 0·95, P for trend=0·0244). However, there was no association between folate and diabetes among men.
Table 2 Type 2 diabetes (T2D) according to tertiles (T) of energy-adjusted folate intake (Incidence rate ratios (IRR) and 95 % confidence intervals)
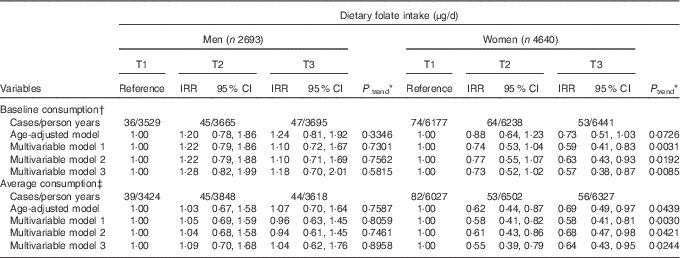
AHEI, Alternative Healthy Eating Index.
* P values for linear trend were obtained by imputing the median value of each tertile and treating it as a continuous variable using a modified Poisson regression with a robust error estimator.
† The multivariable model 1 for baseline consumption, the multivariable model 1 included age (years), higher education level (yes/no), regular exercise (yes/no), waist circumference (cm), total energy intake (kJ/d) and baseline fasting blood glucose (mg/dl) in both men and women and additionally adjusted for smoking status (never/former/current) in men and current smoking (yes/no), alcohol consumption (g/d) in women. In addition to model 1, the model 2 included animal Fe (mg/d), glycaemic load and Mg intake (mg/d) in both men and women and additionally adjusted for fibre from cereals and grains (g/d) for women. The mode1 3 was additionally adjusted for total vegetables (g/d), dairy products (g/d), sweetened beverages (g/d), coffee consumption (g/d) and AHEI for men and women, as well as variables in the model 2 and additionally adjusted for red meat (g/d) in men.
‡ For average consumption, the multivariable model 1 included age (years), higher education level (yes/no), regular exercise (yes/no), waist circumference (cm) and baseline fasting blood glucose (mg/dl) in both men and women and additionally included smoking status (never/former/current) in men and total energy intake (kJ/d), current smoking (yes/no) and alcohol consumption (g/d) in women. In addition to model 1, the model 2 included Fe form animal food (mg/d), glycaemic load and Mg intake (mg/d) in both men and women and additionally adjusted for fibre from cereals and grains (g/d) for women. The mode1 3 was additionally adjusted for total vegetables (g/d), dairy products (g/d), sweetened beverages (g/d), coffee consumption (g/d) and AHEI for men and women, as well as variables in the model 2 and additionally adjusted for red meat (g/d) in men.
Table 3 shows the associations of dietary folate intake with T2D incidence in NFG and IFG group. Higher dietary intake of folate was associated with lower T2D risk both in NFG (IRR=0·32; 95 % CI 0·13, 0·81, P for trend=0·0118 for baseline dietary folate intake) and IFG group (IRR=0·61; 95 % CI 0·37, 0·98, P for trend=0·0382 for average dietary folate intake) among women (P for interaction=0·0917 for baseline folate intake; P for interaction=0·7653 for average consumption), but not among men (P for interaction> 0·05).
Table 3 Type 2 diabetes (T2D) according to tertiles (T) of dietary folate in normal fasting glucose (NFG) and impaired fasting glucose (IFG) group (Adjusted incidence rate ratios (IRR) and 95 % confidence intervals)
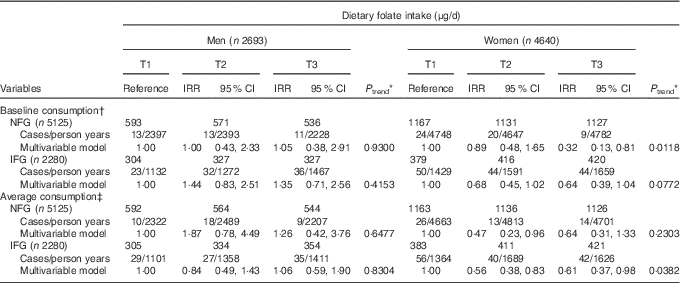
KAHEI, Korean Alternative Healthy Eating Index.
* P values for linear trend were obtained by imputing the median value of each tertile and treating it as a continuous variable using a modified Poisson regression with a robust error estimator.
† The multivariable model for baseline dietary folate consumption was adjusted for age (years), higher education level (yes/no), smoking status (never/former/current), regular exercise (yes/no), waist circumference (cm), total energy intake (kJ/d), baseline fasting blood glucose (mg/dl), Fe form animal food (mg/d), glycaemic load, Mg intake (mg/d), total vegetables (g/d), dairy products (g/d), red meat (g/d), sweetened beverages (g/d), coffee consumption (g/d) and KAHEI in men and for age (years), higher education level (yes/no), current smoking (yes/no), alcohol consumption (g/d), regular exercise (yes/no), waist circumference (cm), total energy intake (kJ/d), baseline fasting blood glucose (mg/dl), Fe form animal food (mg/d), glycaemic load, Mg intake (mg/d), fibre from cereals and grains (g/d), total vegetables (g/d), dairy products (g/d), sweetened beverages (g/d), coffee consumption (g/d) and KAHEI in women.
‡ The multivariable model for average dietary folate consumption was adjusted for age (years), higher education level (yes/no), smoking status (never/former/current), regular exercise (yes/no), waist circumference (cm), baseline fasting blood glucose (mg/dl), Fe form animal food (mg/d), glycaemic load, Mg intake (mg/d), total vegetables (g/d), dairy products (g/d), red meat (g/d), sweetened beverages (g/d), coffee consumption (g/d) and KAHEI in men and for age (years), higher education level (yes/no), current smoking (yes/no), alcohol consumption (g/d), regular exercise (yes/no), waist circumference (cm), total energy intake (kJ/d), baseline fasting blood glucose (mg/dl), Fe form animal food (mg/d), glycaemic load, Mg intake (mg/d), fibre from cereals and grains (g/d), total vegetables (g/d), dairy products (g/d), sweetened beverages (g/d), coffee consumption (g/d) and KAHEI in women.
Consumption of multinutrients and/or vitamins supplements was more prevalent in the higher tertile groups of both baseline and average folate consumption (Table 1 and online Supplementary Table S1), and supplement users might have special behaviours affecting their risk of developing de novo diabetes. Therefore, we further analysed the association between dietary folate intake and risk of developing diabetes after excluding users of multinutrients or multinutrients and/or vitamins (Table 4). Both baseline and average dietary folate intake were inversely associated with T2D in multinutrient non-users (IRR=0·60; 95 % CI 0·39, 0·93; P for trend=0·0213 in T3 for baseline consumption; IRR=0·65; 95 % CI 0·43, 0·996; P for trend=0·0401 in T3 for average consumption) among women. There was also an inverse association between dietary folate intake and T2D incidence in multinutrient and/or vitamin non-users (P for trend=0·0453 for baseline consumption and 0·0786 for average consumption) among women, although the result was not statistically significant in the analysis using average consumption.
Table 4 Type 2 diabetes (T2D) according to tertiles (T) of energy-adjusted folate intake among supplement non-users (Adjusted incidence rate ratios (IRR) and 95 % confidence intervals)
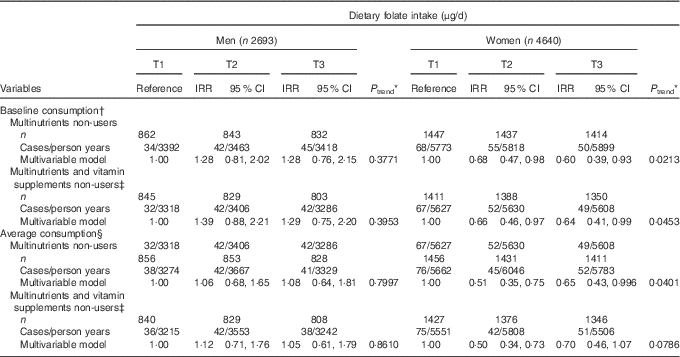
KAHEI, Korean Alternative Healthy Eating Index.
* P values for linear trend were obtained by imputing the median value of each tertile and treating it as a continuous variable using a modified Poisson regression with a robust error estimator.
† The multivariable model for baseline dietary folate consumption was adjusted for age (years), higher education level (yes/no), smoking status (never/former/current), regular exercise (yes/no), waist circumference (cm), total energy intake (kJ/d), baseline fasting blood glucose (mg/dl), Fe form animal food (mg/d), glycaemic load, Mg intake (mg/d), total vegetables (g/d), dairy products (g/d), red meat (g/d), sweetened beverages (g/d), coffee consumption (g/d) and KAHEI in men and for age (years), higher education level (yes/no), current smoking (yes/no), alcohol consumption (g/d), regular exercise (yes/no), waist circumference (cm), total energy intake (kJ/d), baseline fasting blood glucose (mg/dl), Fe form animal food (mg/d), glycaemic load, Mg intake (mg/d), fibre from cereals and grains (g/d), total vegetables (g/d), dairy products (g/d), sweetened beverages (g/d), coffee consumption (g/d) and KAHEI in women.
‡ Vitamin supplements were supplements of vitamin A, β-carotene, vitamin C, vitamin E and vitamin B complexes.
§ The multivariable model for average dietary folate consumption was adjusted for age (years), higher education level (yes/no), smoking status (never/former/current), regular exercise (yes/no), waist circumference (cm), baseline fasting blood glucose (mg/dl), Fe form animal food (mg/d), glycaemic load, Mg intake (mg/d), total vegetables (g/d), dairy products (g/d), red meat (g/d), sweetened beverages (g/d), coffee consumption (g/d) and KAHEI in men and for age (years), higher education level (yes/no), current smoking (yes/no), alcohol consumption (g/d), regular exercise (yes/no), waist circumference (cm), total energy intake (kJ/d), baseline fasting blood glucose (mg/dl), Fe form animal food (mg/d), glycaemic load, Mg intake (mg/d), fibre from cereals and grains (g/d), total vegetables (g/d), dairy products (g/d), sweetened beverages (g/d), coffee consumption (g/d) and KAHEI in women.
Discussion
To our knowledge, this prospective cohort study is the first study to evaluate the association between folate intake and the risk of developing diabetes. During the follow-up period, there was the inverse association between dietary folate intake and diabetes incidence risk among women. These inverse associations were found in both NFG group and IFG group among women.
There was no prospective cohort study to be directly compare with our finding of an inverse association between dietary folate intake and T2D risk, although several studies using folic acid supplementation( Reference Solini, Santini and Ferrannini 31 ) with high doses of folic acid comparing to dietary intake. A meta-analysis reported that B vitamin supplementation including folate did not reduce the risk of vascular diseases( Reference Bailey, da Silva and West 9 ). However, a short-term intervention study in healthy overweight participants (n 60) revealed that folic acid supplementation (2·5 mg/d for 3 months) reduced FBG level and improved insulin sensitivity( Reference Solini, Santini and Ferrannini 31 ). The improvements of insulin resistance and endothelial dysfunction were accompanied by reduced Hcy level after 6 months of folic acid administration (5 mg/d) with vitamin B12 (500 μg/d) in T2D patients (n 50)( Reference Setola, Monti and Galluccio 32 ). In a meta-analysis of 183 diabetic patients from four studies, folic acid supplementation was also associated with better glycaemic control than placebo treatment( Reference Sudchada, Saokaew and Sridetch 33 ). MTHFR is the key rate-limiting enzyme required for the conversion of dietary folate to 5-methyltetrahydrofolate which is the methyl group donor required to convert Hcy to methionine in vivo ( Reference Jacques, Bostom and Williams 34 ). A meta-analysis of studies on T2D associated with Hcy and the MTHFR and mendelian randomisation of the MTHFR gene suggested that the potential gene-environment interactions involved in the development of T2D and that the associations of Hcy with T2D may be modified by environmental factors such as dietary folate intake( Reference Huang, Ren and Huang 18 ). The results of our prospective observational study among adult participants without T2D diagnosed by physician or FBG (≥126 mg/dl; 7·0 mmol/l) (n 7333) were consistent with the results of the above intervention study, although some but not all trials have revealed a significant beneficial effect of folic acid supplementation on glucose metabolism( Reference Song, Cook and Albert 35 ).
Two different dietary folate intakes, the baseline and the average folate consumption were used in the analyses. The average folate consumption was calculated with intake data from the baseline until just before the censoring time or end point to reduce measurement error and/or to better represent long-term dietary patterns( Reference Hu, Stampfer and Rimm 36 ). In the present study, it was difficult to assert which intake of baseline and average folate was better associated with T2D incidence and whether averaging folate intakes reduced measurement error. Therefore, further prospective cohort studies using repeated measures of dietary folate are needed to confirm this inverse relationship of dietary folate intake.
T2D is a chronic non-communicable disease characterised by β-cell failure in the setting of insulin resistance. Although the mechanisms which folate reduces the risk of diabetes are not clearly understood, one possible mechanism is that folate can reduce the level of Hcy, which may inhibit the insulin-stimulated tyrosine phosphorylation of the insulin receptor β-subunit and its substrates, reduce the activity of the p85 regulatory subunit of phosphatidylinositol 3-kinase, and reduce insulin-stimulated glycogen synthesis( Reference Najib and Sanchez-Margalet 37 ). Furthermore, it was reported that Hcy inhibits the activation of adenylate cyclase and protein kinase C pathways through induction of insulin secretion in pancreatic β cells( Reference Patterson, Flatt and Brennan 38 ). In addition, folate has been shown to ameliorate arsenic- and nicotine-induced oxidative damage in rat pancreatic tissue( Reference Mukherjee, Das and Mukherjee 39 , Reference Bhattacharjee, Prasad and Pal 40 ). The main circulating metabolite of folate, 5-methyltetrahydrofolate, has prominent antioxidant activity( Reference Rezk, Haenen and van der Vijgh 41 ) and increases nitric oxide production( Reference Stroes, van Faassen and Yo 42 ). Increased production of reactive O2 species from mitochondrial overstimulation and reactive nitrogen species from excess nitric oxide generation in β cells may inhibit the electron transport chain and thus reduce energy production, damage DNA and promote the formation of advanced glycation end products, ultimately leading to β cell dysfunction( Reference Cerf 43 ). These findings may suggest that folate itself may influence the risk of diabetes, independent of its regulation of Hcy concentration.
Although we could not define prediabetes, it is worth to note the inverse association between dietary folate intake and T2D incidence risk even in IFG group, as well as in NFG group. To our best knowledge, there was no prospective cohort study considering NFG and/or IFG status on the association between folate intake and T2D incidence.
We unexpectedly found the higher FBG level in T3 of folate intake compared with T1. It may be due to the higher proportion of IFG group, which had higher folate intake than NFG group (data not shown; all P values <0·05 for both baseline folate and average folate in men and women), in T3 of dietary folate intake compared with T1. The higher folate intake in IFG was likely because more participants in IFG group have been alerted to high FBG and thus have been already changed their diets compared with NFG group. Furthermore, the increasing pattern of T2D risk in IFG group from T1 of the baseline folate intake to T3 appeared. The likelihood of T2D occurrence among participants with IFG compared with NFG was a predictable result. The increasing pattern from T1 to T3 may be also due to already changed diet among participants with high FBG.
We found the sex difference in the association between dietary folate intake and T2D incidence risk ( P for interaction=0·0411 for baseline folate intake; P for interaction=0·0471 for average folate intake), although foods highly providing dietary folate and contributing variation of total folate intake (R 2) were almost same between men and women (online Supplementary Table S2). In the present study, we could not assert why there was sex difference in the association between dietary folate intake and T2D incidence risk. However, it may be partially explained by that the higher concentration of serum Hcy, the lower concentration of serum folate and vitamin B12 among men than women reported in our previous study among adults in a community of the present study areas( Reference Kim, Kim and Kim 44 ), although dietary folate intake was higher among men than women. Another previous study on B vitamins and Hcy level among Hispanic men and women suggested the possibility that the higher concentration in men even though controlling for dietary B vitamins intakes may be due to sex differences in Hcy metabolic regression and that the higher intake of B vitamins including folate may be needed to lower Hcy in men than in women( Reference Kim, Ordovas and Selhub 45 ).
There were some limitations to the present study. This study was an observational prospective cohort study and the development of diabetes may be influenced by lifestyle changes( Reference Tuomilehto, Lindström and Eriksson 46 ). We included many potential confounders correlated with T2D( Reference Agardh, Allebeck and Hallqvist 12 – Reference Ley, Hamdy and Mohan 17 , Reference McCullough, Feskanich and Stampfer 25 ). However, there may be residual confounding by unmeasured confounding factors such as Hcy level. In addition, confounding from multiple food sources was still possible. Because higher daily intake of folate tended to be related to some food that may have beneficial effects on T2D such as green leafy vegetable, whole grain and dairy products (online Supplementary Fig. S1). Therefore, the results from the present study should be interpreted in the context of the whole dietary patterns. Second, we could not confirm the association between folate and T2D with biomarker such as serum/erythrocyte folate. Third, follow-up loss may lead to biased results. Although participants who retained until the second revisit were likely to be relatively healthier comparing to participants who lost to follow-up after baseline and the first revisit, the findings from the analyses using only data until the first revisit were very similar to this study (data not shown). Furthermore, in the sensitivity analysis of the assignment methods of follow-up time to evaluate the effect of follow-up losses in the exposure group (online Supplementary Table S3); (1) the smallest denominator and (2) the largest denominator, the significant inverse associations appeared in both assignment methods of follow-up time. However, the problem resulting from the possibility that loss to follow-up was biased with respect to both exposure and outcome remains( Reference MacMahon and Pugh 23 ). It is generally recognised that the validity of the statistical results derived from the study (such as risk ratios) may be affected when follow-up losses are uneven in both the exposure and outcome categories( Reference Greenland 47 ). Thus, we also conducted household visit survey among subjects who did not participate in repeated examinations between 2015 and 2016 to check if there was a new T2D diagnosed by medical doctor and if taking medication. The follow-up period was different from the present study and T2D by FBG could not be included. Considering non-participants in repeated examination (follow-up method in the present study) as loss to follow-up( Reference Greenland 47 ), there was no significant follow-up bias according to tertiles of dietary folate intake (sex and age standardised follow-up bias, P=0·2501 in the entire participants; P=0·3809 in NFG group; P=0·0646 in IFG group). However, cohort studies following up over a longer period are traditionally subject to selection bias. This implies participants who are at risk are more likely to participate and continue being in the study. Even when the results are valid for the present cohort and present conditions, selection bias may influence the external validity and generalisability of the results. Fourth, self-report of physician-diagnosed diabetes and medication was not validated with medical record, although self-reported T2D was consistently responded between visits (92·4 %). In addition, the glycosylated Hb (%) and the OGTT was not measured in the cohort and thus diabetes by OGTT could not be defined. Finally, there was limitation directly to explain the possible mechanism under the development of T2D without biomarkers such as folate and vitamin B12. Further long-term prospective studies with repeated measures of biomarkers are needed.
In conclusion, higher intake of folate may be associated with a lower risk of diabetes. In addition, this effect is independent of previously identified risk factors of diabetes and other nutrient factors. Further epidemiological and experimental studies are required to confirm the effect of folate on diabetes risk.
Acknowledgements
The authors thank the Korea Centers for Disease Control and Prevention for the administration of data.
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2004-E71004-00, 2005-E71011-00, 2006-E71009-00, 2007-E71002-00, 2008-E71004-00, 2009-E71006-00, 2010-E71003-00, 2011-E71002-00, 2012-E71007-00, 2013-E71008-00) and was supported by the National Research Foundation of Korea grant funded by the Korea government (Ministry of Science, ICT & Future Planning) (no. 2016R1A2B2011352).
S. M. H. and H. W. W equally contributed to the design of this study and the data analysis and wrote the manuscript. S. Y. K. analysed data and was involved in interpreting the results. B.-Y. C., Y.-H. L., D. H. S., M.-H. S. B.-Y. C., and B. Y. C. were involved in data collection and management. M. K. K. designed the study and supervised all aspects of its implementation. All authors helped to conceptualise the study, interpret findings and review drafts of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517003087







