The high rate of morbidity and mortality associated with neural tube defects (NTD) raises important public health concerns, especially in India, where NTD incidence of 6·57–8·21 per 1000 live births was reported ina door-to-door survey in Balrampur district of Uttar Pradesh, which is very high compared with rest of the world( Reference Cherian, Seena and Bullock 1 ). However, the hospital-based prevalence rate of anencephaly, spina bifida and encephalocele, from forty-six regional registries spread all over the country, was reported to be 1·15, 1·28 and 0·28 per 1000 total births that includes live births, stillbirths and termination of pregnancy( 2 ). Being multifactorial, various environmental and genetic factors are involved in increasing the risk of NTD and the impact varies in different populations.
Socio-cultural and nutritional factors are important variables in the aetiology, prevalence and distribution of such developmental diseases. Epidemiological studies have correlated the risk of NTD with low socio-economic status and nutritional intake( Reference Munoz, Lacasana and Aburto 3 – Reference Carmichael, Yang and Feldkamp 5 ). Deficiency of folate and vitamin B12 disrupts the one-carbon metabolic cycle, which leads to the accumulation of homocysteine and in turn may cause NTD through various mechanisms like impaired methylation activity, endothelial dysfunction through oxidative stress, and teratogenic interaction of increased homocysteine with the N-methyl-d-asparate receptor system involved in neuronal development and migration( Reference Refsum 6 ). The breakthrough discovery of lower NTD risk with folate or multivitamin supplementation or food fortification proved to be helpful in reducing the incidence of NTD in many parts of the world( Reference Czeizel and Dudas 7 – Reference Wilson, Johnson and Wyatt 11 ). In India however, especially among lower socio-economic communities, the incidence is still on rise, which may be attributed to the absence of fortified food and limited exposure to early antenatal care. It can therefore be assumed that preventive interventions such as periconceptional supplementation with folic acid/other vitamins or prenatal screening are not widespread in these communities. Moreover, general food items that are consumed on a day-to-day basis are the major contributors to various nutritional requirements. The compound effects of food consumption and their interactions cannot be assessed by focusing on one or two nutrient/micronutrient deficiencies in the maternal body during pregnancy. It is therefore important to expand our understanding on the contribution of dietary foods in the aetiology of NTD. Studies have shown that NTD risk is associated with low consumption of meat, milk, fresh vegetables and fruits( Reference Zhang, Xin and Gu 12 , Reference Yin, Xu and Xu 13 ); however, dietary patterns have ethnic and geographic variations. Additionally, the contaminants present in drinking water are also known to pose a risk for developmental defects( Reference Nieuwenhuijsen, Martinez and Grellier 14 ).
Keeping in view the above issues, the present study aimed to investigate the association between sociodemographic and nutritional factors (maternal age, education, migration, dietary intake, etc.) and NTD among the North Indian population.
Materials and methods
A case–control study was carried out in four government hospitals of Delhi, India, after obtaining formal ethical clearance. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the Institutional Ethical Committee following ethical guidelines for biomedical research on human subjects of the Indian Council of Medical Research (http://www.icmr.nic.in/ethics_SOP.pdf). The study was conducted from January 2008 to August 2010. Diagnosis of children with NTD was done by the doctors of the hospitals concerned, from live births, stillbirths/terminations and antenatal diagnosis by ultrasonography. The inclusion criterion for cases was being the mother of a child with NTD and inclusion criteria for the control group were being the mother of at least one healthy child, with no previous history of unsuccessful pregnancy or abnormal conceptions. The control mothers were matched with the case mothers for age group (±3 years), community and locality of residence, in the ratio of 1:2 (cases to controls).
During the study period, 308 case mothers of NTD children were interviewed; however, controls could be matched for only 284 cases. Therefore, twenty-four cases were excluded from the study. Once the case and control mothers had agreed to participate in the study and gave their written consent, personal interviews were conducted by trained field investigators. The interview schedule included information on various demographic parameters such as maternal age, socio-economic status (family income, education and occupation), religious community (Hindu, Muslim, Sikh, and Christian), migration history, and frequency of consuming common food groups (daily or weekly) during the period from 3 months before pregnancy to the first 3 months of pregnancy. Socio-economic class was calculated according to the modified Kuppuswamy scale( Reference Parasher 15 ) based on family income, education and occupation. To address the effect of different NTD phenotypes, the cases were segregated into two groups: upper NTD (anencephaly and encephalocele) and lower NTD (spina bifida), based on the position of the defect; the case mothers of children with multiple NTD were excluded for this part of the analysis.
The χ 2 test or Fisher's exact test was applied on categorical variables. Binary logistic modelling (univariate and multivariate) and calculation of odds ratios with 95 % confidence intervals were carried out to evaluate the effect of risk factors on the incidence of NTD, using the statistical software package SPSS version 17·0. A P value of <0·05 for univariate and <0·01 for multivariate analysis was considered as statistically significant.
Results
Sociodemographic characteristics
The cases were composed of different types of NTD; the majority were found to be spina bifida (42 %; n 119), followed by anencephaly (40 %; n 114) and encephalocele (11 %; n 32). The remaining 7 % (n 19) of cases had multiple NTD. The general characteristics of the cases included and excluded from the study are presented in Table 1 and suggest no statistically significant differences between the two groups. As shown in Table 2, only 25 % of the NTD cases were live births and remaining were stillbirths or terminated. The sex ratio (female:male) was 1·13 for the case infants and 1·03 for control infants; however, the sex could not be recorded for 31 % of cases and 7 % of controls, as the data were collected during the antenatal period, after the diagnosis of an NTD child through ultrasonography. The mean ages at conception of case mothers (24·4 (sd 4·2) years) and controls (24·5 (sd 3·5) years) were found not to be statistically different (t = 1·02; df = 886; P > 0·05), whereas the distribution according to age group showed a statistically significant difference (P < 0·05), with more mothers in the younger (<21 years) and fewer mothers in the older age group (>30 years) in the cases compared with the controls. Borderline statistical significance with respect to paternal education was observed on applying the χ 2 test between case and control groups (P = 0·05). The distribution of household socio-economic status showed higher incidence of NTD among the lower classes compared with the middle classes.
Table 1 General and sociodemographic characteristics of cases included and excluded from the study, Delhi, India, January 2008 to August 2010
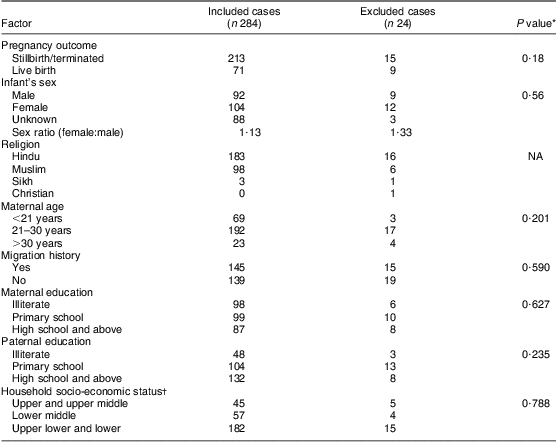
NA, not applicable.
*Calculated through the χ 2 test.
†Calculated according to the modified Kuppuswamy scale.
Table 2 General and sociodemographic characteristics of case and control groups, Delhi, India, January 2008 to August 2010
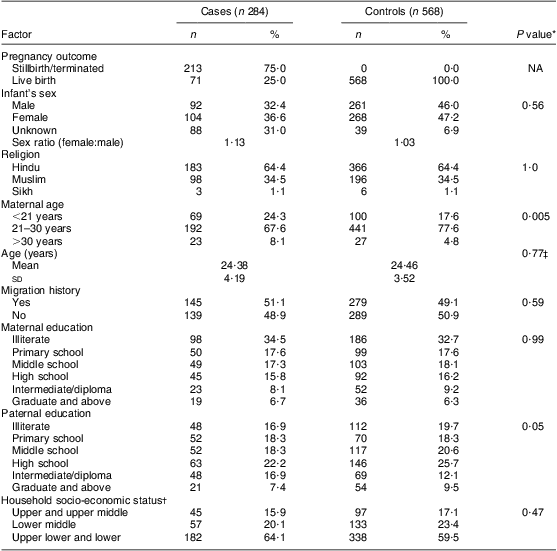
NA, not applicable.
*Calculated through the χ 2 test.
†Calculated according to the modified Kuppuswamy scale.
‡Calculated through the t test.
Risk analysis
Table 3 shows the nutritional profile of case and control mothers during the periconceptional period. It was found that more case mothers were using groundwater for drinking purposes and unpasteurized milk compared with control mothers, thereby increased risk for NTD was observed (OR = 1·54, 95 % CI 1·04, 2·95, P = 0·03 and OR = 1·53, 95 % CI 1·13, 2·08, P = 0·01, respectively). More case mothers than control mothers consumed less than 100 ml milk/d, although the difference was not statistically significant. Investigating the frequency of consumption of meals per day, and vegetables, legumes and fresh fruit per week, no association was observed with the risk for NTD-affected pregnancies. It was further observed that more of the case mothers were vegetarian, although the difference between case and control mothers was found to be borderline statistically significant (P = 0·06). Folic acid supplementation was not found to be statistically significant on applying the χ 2 test (P = 0·15).
Table 3 Nutritional profile of case and control mothers in the periconceptional period, Delhi, India, January 2008 to August 2010
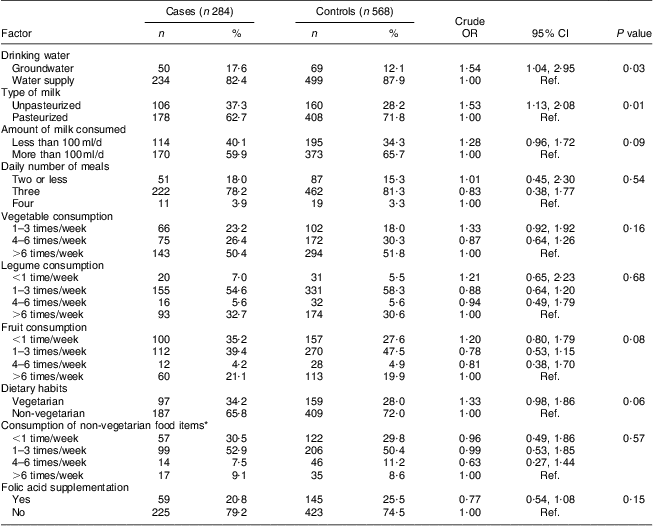
Ref., reference category.
*Among non-vegetarians.
On applying multivariable conditional logistic modelling using the backward stepwise procedure, consumption of unpasteurized milk (adjusted OR (AOR) = 1·45, 95 % CI 1·07, 1·99; P = 0·02), vegetables less than three times weekly (AOR = 1·47, 95 % CI 1·01, 2·17; P = 0·04) and fruits less than once weekly (AOR = 1·27, 95 % CI 0·95, 1·92; P = 0·05), and vegetarian dietary habits (AOR = 1·34, 95 % CI 0·99, 1·83; P = 0·05), were found to be borderline significantly associated with the risk of NTD (Table 4). In order to address the phenotypic heterogeneity, the NTD cases were further segregated into two groups: upper NTD (anencephaly and encephalocele) and lower NTD (spina bifida). The multivariable logistic model was applied on both groups separately, and compared against all control mothers. It was found that groundwater use (P = 0·02) and vegetable consumption less than three times weekly were associated (P = 0·05; borderline statistical significance) with risk only for lower NTD, whereas unpasteurized milk use (P = 0·01), milk consumption less than 100 ml/d (P = 0·02) and vegetarian dietary habits (P = 0·03) were likely to increase the risk for upper NTD.
Table 4 Multivariate logistic regression results on risk factors for neural tube defects (NTD), upper NTD and lower NTD, Delhi, India, January 2008 to August 2010
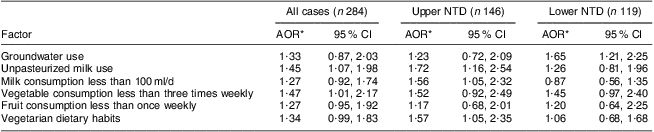
*AOR, adjusted odds ratio (adjusted for socio-economic status and maternal age).
Discussion
The results of the present study demonstrate evidence for the association of demographic factors and nutritional indicators with NTD. The higher incidence of case mothers in the lower (<21 years) and higher (>30 years) age groups, as compared with control mothers, is consistent with the study of Golalipour et al.( Reference Golalipour, Najafi and Keshtkar 16 ), while some studies have shown increased risk with higher or lower maternal age group( Reference Wen, Ethen and Langlois 17 , Reference Zeyrek, Soran and Cakmak 18 ). Various studies have measured social indicators at the individual and community level and found higher risk of NTD in the children of women with lower educational levels, lower incomes and employment( Reference Rosano, Del Bufalo and Burgio 19 , Reference Canfield, Ramadhani and Shaw 20 ). The present study revealed higher incidence of NTD among women belonging to lower and upper lower socio-economic classes than in their middle-class counterparts. The relationship of lower socio-economic status with NTD may also reflect low dietary consumption, access to medical care and/or direct knowledge about the importance of folate in the diet.
Contaminants (like nitrates) or disinfectants (like chlorine, disinfectant by-products) in drinking water are exposed to diverse physical and chemical agents that can cause adverse health effects and result in various birth defects including congenital cardiac defects and NTD( Reference Cedergren, Selbing and Lofman 21 – Reference Hwang and Jaakkola 24 ). Various studies also reported in 2011 that agricultural activities in the northern or central part of India resulted in a high concentration of nitrate or fluoride in groundwater, making it unsafe for drinking( Reference Reddy, Nagabhushanam and Peters 25 , Reference Suthar 26 ). Moreover, a study in 2010 reported high nitrate concentration in groundwater in the study area of Delhi( Reference Dash, Sarangi and Singh 27 ) and it was observed in the present study that more case mothers had migrated from rural areas of northern and central parts of India to Delhi. This is reflected in the fact that the women in our study who used more groundwater for drinking, which involved potential exposure to contaminants, had a higher risk of having children with NTD.
Milk has been considered one of the most natural and highly nutritive parts of a daily balanced diet; however, unpasteurized milk contains various pathogens (Gram-positive or Gram-negative bacteria, viruses) which lower the immune response. The consumption of unpasteurized milk is most commonly found to be associated with listeriosis, an infection, resulting in poor pregnancy outcomes( Reference Ross, Jones and Lynch 28 ). The significant difference observed between case and control mothers with respect to the type of milk they used suggests that mothers who consume unpasteurized milk (domestic or provided by a milkman) are at higher risk for NTD, more specifically upper NTD, than those who consume pasteurized milk (packet milk). However, no other study has reported any correlation with birth defects, although the consumption of unpasteurized milk is not recommended during pregnancy( Reference Janakiraman 29 ). Therefore it can be hypothesized that unpasteurized milk consumption by the pregnant mother may lead to NTD in the developing embryo; however, it is difficult to provide any biological explanation at this point.
The interactive effect of the micronutrients (folate and vitamin B12) that are necessary for the proper development of an embryo can indirectly be assessed by studying the role of food items during early pregnancy. The current study suggested an association of NTD risk with low consumption of vegetables, fruits and milk, which is consistent with previous studies( Reference Zhang, Xin and Gu 12 , Reference Yin, Xu and Xu 13 ). However, the present study's finding of a significant association of NTD occurrence among mothers with vegetarian dietary habits provides a clue towards the role of micronutrients which may be low in a vegetarian diet. Being a country predominated by vegetarians, in India a high prevalence of vitamin B12 deficiency has been reported in pregnant mothers( Reference Pathak, Kapil and Yajnik 30 ) and the parents of NTD neonates( Reference Ratan, Rattan and Pandey 31 ). A previous finding also suggested a role of vegetarian dietary habits in the aetiology of NTD among the Hindu community of India( Reference Deb, Arora and Meitei 32 ). The Mediterranean dietary pattern, characterized by high intakes of fruits, vegetables, vegetable oils, alcohol, fish, legumes and cereals, and low intakes of potatoes and sweets, was found to be correlated with higher levels of serum and red-blood-cell folate, serum vitamin B12 and lower plasma homocystiene, and subsequently decreased risk for NTD( Reference Carmichael, Yang and Feldkamp 5 , Reference Vujkovic, Steegers and Looman 33 ).
In the present study, some case mothers were not included due to the absence of properly matched control mothers; however, no statistical difference was observed between the general characteristics of cases included and excluded from the study. Moreover, the possibility of recall bias is also very limited as the interview from case and control mothers was conducted during pregnancy or immediately after childbirth; also, as the women belonged to low-socio-economic strata, there is consistency in their dietary intake irrespective of their pregnancy status. However, the study has some limitations; the assessment of the contaminants present in the groundwater could not be done, as the localities of the participants varied widely and the collection of water samples was beyond the scope of the study. Further, in the absence of precise nutritional deficiency data (biochemical analysis), the study provides better understanding on the role of general food items and other demographic factors possibly having an impact on the incidence of NTD, in Indian context.
Conclusions
Various nutritional factors – source of water, kind of milk, amount of milk and number of meals in a day, weekly consumption of vegetables, pulses and fruits, dietary habits and weekly consumption frequency of non-vegetarian food items (and/or eggs) – have been investigated in the present study. The findings demonstrated possible association of different types of NTD with the consumption of water, milk, vegetables and fruits and dietary pattern. These dietary food items provide essential nutrients like folic acid, vitamin B12 and other vitamins/minerals required during embryogenesis, and their deficiency may cause NTD in the growing fetus. It was further observed that folic acid supplementation alone was not sufficient to control the births of NTD children in the studied populations. Therefore, women of reproductive age, specifically in the low socio-economic strata, need to be counselled about the importance of necessary modifications in their dietary intake before and during the pregnancy, with the aim towards the prevention of such devastating birth defects. Moreover, many Indian women follow vegetarian dietary habits, which may lead to a deficiency of vitamin B12; therefore, additional supplements to such communities may help in reducing the incidence of NTD. Furthermore we suggest that periconceptional intake of multivitamins (folic acid/vitamin B12/choline), possibly through government-initiated nutritional programmes, might be an effective measure to decrease the NTD incidence in the present population.
Acknowledgements
Sources of funding: The study was supported by the Indian Council of Medical Research, Government of India, Delhi. Conflicts of interest: J.A. is the recipient of the senior research fellowship in the funded research project. The authors declare no conflicts of interest. Authors’ contributions: R.D., K.N.S. and A.K.K. contributed to the conceptualization of the idea. J.A. supported R.D. for data collection, statistical analysis, literature review and manuscript writing. Acknowledgements: The authors are grateful to all participants and hospitals for providing valuable information.






