With the increase in ageing population, osteoporosis has become a major public health problem worldwide that requires the development of a novel approach for its prevention and treatment(Reference Banu, Varela and Fernandes1). In particular, postmenopausal women are of high risk of developing osteoporosis due to the significant alterations in bone metabolism associated with oestrogen deficiency. The rapid bone loss and the increase in bone fragility during menopause have led to the increase in incidence rate of fractures of vertebrae in the spine, hips and wrists among postmenopausal women(Reference Khosla2, Reference Al-Anazi, Qureshi and Javaid3). Hormone replacement therapy was a major regimen for the management of postmenopausal osteoporosis in the past. However, with the report of the increased risk in developing stroke and breast cancer associated with its use(Reference Gass and Dawson-Hughes4), there is a strong demand to develop affordable alternative approaches with minimal side effects for the management of postmenopausal osteoporosis. This is particularly important for ageing populations in developing countries, as the majority of patients could not afford long-term use of high-cost medications.
Dietary sources of phyto-oestrogens (plant-derived compounds that have oestrogenic properties) are increasingly popular due to their potential beneficial effects on human health(Reference Cassidy5). Recent reports indicated that phyto-oestrogens can attenuate bone loss associated with oestrogen deficiency in both animal and human studies(Reference Banu, Varela and Fernandes1, Reference Al-Anazi, Qureshi and Javaid3). Phyto-oestrogens with demonstrated bone-protective effects include soya isoflavones(Reference Zhang, Chen and Lai6, Reference Zhang, Li and Wan7), lignans(Reference Lampe8) and flavonoids(Reference Mok, Chen and Lai9, Reference Pang, Wang and Mok10). Experimental studies(Reference Zhang, Li and Wan7) showed that a diet containing soya extract (Novasoy), but not purified genistein, significantly preserved trabecular bone mass, bone volume and trabecular bone separation in the proximal tibial metaphysis of OVX mice. In addition, high dietary phyto-oestrogen intake was found to be associated with higher bone mineral density (BMD) in postmenopausal but not premenopausal women, suggesting that the positive effects of phyto-oestrogen on bone are influenced by the hormonal status of the individuals(Reference Mei, Yeung and Kung11).
Furthermore, recent studies indicated that Chinese herbs that are traditionally used for the management of bone diseases exert oestrogen-like activities on bone(Reference Zhang, Li and Li12–Reference Zhang, Li and Wan14) and might serve as a potential source of phyto-oestrogens for the management of postmenopausal osteoporosis.
The dried rhizome of Drynaria fortunei (Kunze) J. Smith (Polypodiaceae) (DF) is a Chinese herb traditionally used for the treatment of bone fractures or related diseases(Reference Zhou15). Its Chinese name is ‘Gu Sui Bu’, which means bone fractures healer. Accumulating evidence demonstrated the bone-protective effects of DF in both cell cultures and animal studies(Reference Sun, Dong and Lin16–Reference Guo, Wang and Wang24). Its crude extract has been shown to promote osteoblast differentiation and mineralisation in pre-osteoblastic MC3T3-E1 cells(Reference Jeong, Lee and Yoon17, Reference Jeong, Lee and Yoon18, Reference Chen, Ding and Tang23), increase the proliferation of human osteoprecursor 1 cells(Reference Jeong, Yoon and Kim19) as well as inhibit in vitro bone resorption in mouse osteoclasts(Reference Jeong, Kang and Youn25). In addition, administration of DF crude extract (6·6 mg/d) to 8-week-old BALB/c male mice for 5 weeks increased bone volume/tissue volume by 6·5 %, trabecular number by 10 % and decreased trabecular separation by 9·7 %. DF crude extract was also shown to induce bone formation on the margin of defects created in the parietal bone of New Zealand white rabbits(Reference Wong, Rabie and Bendeus21). Furthermore, the total flavonoid fraction of DF (DFTF) was shown to significantly restore ovariectomy (OVX)-induced bone loss in rats(Reference Sun and Liu22), while different DF fractions were found to increase BMD of the femur head in OVX mice(Reference Wang, Wang and Zhang26).
Phytochemical analysis of DF extract indicated that flavonoids(Reference Wang, Wang and Zhang26, 27–Reference Wang, Zhen and Zhang29) and phenylpropanoids(Reference Wang, Wang and Gao28, Reference Wang, Wong and Yao30) were the active ingredients that account for its osteoprotective activities. One of the highly abundant flavanoid compounds identified in DF is naringin, which is also the major bone-protective ingredient found in citrus fruits(Reference Lu, Zhang and Bucheli31). Naringin was shown to improve bone quality in retinoic acid-induced osteoporosis rats(Reference Wei, Yang and Li32) and orchidectomised rat(Reference Mandadi, Ramirez and Jayaprakasha33) models. Moreover, the direct effects of naringin on proliferation and differentiation of bone cells were demonstrated in murine osteoblast-like MC3T3-E1 cells(Reference Wu, Fong and Tsai34, Reference Li, Zeng and Cai35) and rat osteoblast-like UMR-106 cells(Reference Xie, Wu and Lai36, Reference Wong and Rabie37). Our recent study also reported that naringin was effective in protecting against OVX-induced bone loss in mice and that the stimulatory effects of naringin on osteoblastic functions in UMR-106 cells were oestrogen receptor (ER) dependent(Reference Xie, Wu and Lai36), suggesting that it might act as a phyto-oestrogen in bone tissue.
The present study aimed to systematically characterise the dose-dependent effects of DFTF on bone properties in OVX mice as well as the oestrogenic effects of DFTF and its isolated compounds on osteoblastic functions in UMR-106 cells. It is hoped that the studies would provide evidence to support the use of D. fortunei as an alternative for the prevention and treatment of postmenopausal osteoporosis.
Experimental methods
Materials
All reagents for cell culture, RT-PCR and plasmid transient transfection kit were purchased from Life Technologies, Inc., unless otherwise indicated. 17β-Oestradiol (E2) was purchased from Sigma-Aldrich. The anti-oestrogen, ICI 182780, was purchased from Tocris Cookson Limited. Primers were obtained from Tech Dragon Limited. Commercial diets were purchased from Teklad.
Preparation of rhizoma Drynaria total flavonoid extract and characterisation of single compounds
Rhizomes of D. fortunei were collected in Guizhou province, China, in 2005, and were identified by Professor Zhou Rong-Han (Chinese Pharmaceutical University, Nanjing, Jiansu, China) and extracted as described previously(Reference Wang, Wang and Zhang26). In brief, rhizomes of DF were cut and extracted with 60 % ethanol to yield 60 % ethanol extract. The extract was further separated by a D-101 macroporous resin column (Φ25 × 150 cm) with gradient elution of water (DFA), 30 % (DFB), 50 % (DFC) and 95 % (DFD) ethanol to yield four different fractions. DFB, DFC and DFD were positive to HCl–Mg powder reaction, which was identified as the flavonoids fraction of DF. They were combined to yield DFTF. Single compounds were isolated through HPLC on a Shimadzu C18 column (Φ250 × 4·6 cm) in an Agilent series 1100 HPLC (Agilent Technologies, Inc.) in the DFB fraction. The peaks of the HPLC chromatogram from DFB were identified as previously described(Reference Wang, Wang and Zhang26). The structure of the three most abundant isolated compounds in DFB are shown in Fig. 1, which are naringin, (2S)-5,7,3′,5′-tetrahydroxy-flavonone 7-O-neohesperidoside (compound 1) and 5,7-dihydroxychromone 7-O-neohesperidoside (compound 2), with a yield of 0·045, 0·134 and 0·022 %. The chemical name of compounds 1 and 2 are (S)-7-(((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-(((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)-2-(3,5-dihydroxyphenyl)-5-hydroxychroman-4-one and 7-(((2S,3R,4S,5S,6R)-4,5-dihydroxy-6-(hydroxymethyl)-3-(((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)-5-hydroxy-4H-chromen-4-one. Three isolated compounds were characterised on the basis of comprehensive analyses of their mono- and bi-dimensional NMR spectra. Naringin(Reference Chang, Lee and Cho38), compound 1(Reference Wang, Wang and Zhang26) and compound 2(Reference Kosuge, Mitsunaga and Koike39) were identified by comparing their spectral data with previously reported data.
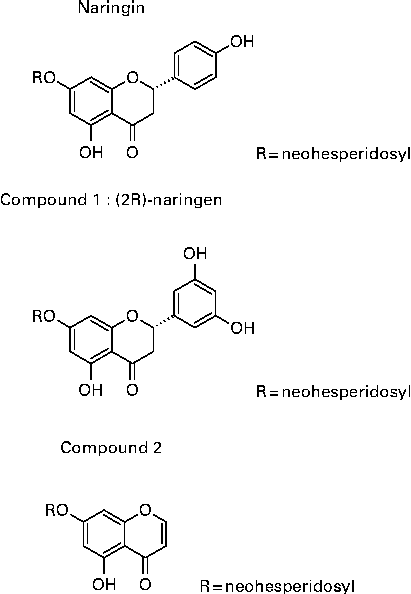
Fig. 1 Structure of Drynaria fortunei total flavonoids (DFTF) isolated compounds. (2R)-Naringin, (2S)-5,7,3′,5′-tetrahydroxy-flavonone 7-O-neohesperidoside (compound 1) and 5,7-dihydroxychromone 7-O-neohesperidoside (compound 2) were isolated from DFTF with the yield of 0·045, 0·134 and 0·022 %, respectively.
Animal care and diet
A total of sixty 1-month-old female C57/BL6J mice (obtained from the Chinese University of Hong Kong) were housed in environmentally controlled central animal facilities. The mice were either sham-operated or OVX at the age of 1 month for simulating an oestrogen-deficient environment. After a 2-week recovery period, the mice were randomly divided into six groups: sham vehicle (2 % ethanol); OVX vehicle; OVX E2 (2 μg/g per d) and three groups of OVX DFTF extract (low dose: 0·087; medium dose: 0·173; and high dose: 0·347 mg/g per d)). They were fed with a control diet with a normal Ca level (0·6 % Ca and 0·65 % P) (TD 98 005; Teklad) 2 d before the treatments. During the treatment period, animals were pair-fed with the control diet, received treatment orally for 6 weeks and were allowed free access to water throughout the course of the studies, as previously described(Reference Xie, Wu and Lai36). The care and treatment protocol were approved by the Animal Ethics Committee of the Hong Kong Polytechnic University.
At 1 d before killing, the mice were placed in metabolism cages. Their urine sample was collected, centrifuged and stored at − 20°C until use. At the end of treatment, the mice were anaesthetised with 10 % ketamine (75 mg/kg, #013004; Alfamedic Limited). Blood samples were taken from the inferior vena cava, followed by the collection of uteri and bone specimens (femur, tibia and lumbar spine). The serum samples of the mice were stored at − 80°C and the left femur, tibia and lumbar spine were stored at − 20°C until analysis.
Biochemical assays of serum and urine samples
Ca and P concentrations of the serum and urine samples were studied by the O-cresolphthalein complexon colour development method and p-methylaminophenol method, respectively. Both biomarkers were measured by commercial kits (Wako Pure Chemical Industries Limited). The urinary excretion of deoxy-pyridinoline (Dpd), a collagen degradation product that reflects bone resorption rate, was measured using a Metra® DPD EIA kit (Quidel) with a microplate reader. Urinary creatinine (Cr) was used as an internal control and was assessed with the Jaffe method using a commercial kit (Wako Pure Chemical Industries Limited). Urinary Ca, P and Dpd levels were expressed as its excretion per unit of Cr (Ca/Cr, P/Cr and Dpd/Cr).
Assessment of bone properties by peripheral quantitative computed tomography
Bone properties of the left femur, tibia and lumbar spine region L1 were measured using StraTec XCT2000 machine (Norland Stratec Medizintechnik, GmbH). Mid-shaft and distal regions of femur and tibia were scanned using a developed protocol designed for studying isolated small bones(Reference Gasser and Willnecker40). The distal/proximal site was defined as 2·5 mm away from femur/tibia head. The mid-shaft was the middle region of each long bone. Total BMD, trabecular BMD, total cross-sectional area, trabecular cross-sectional area and stress–strain index (SSI) in the distal/proximal site were determined.
Biomechanical measures of tibia mid-diaphysis
Mechanical strength of mid-shaft tibia was measured using a specified three-point bending machine (Hounsfield Test Equipment), as previously described(Reference Zhang, Leung and Che41). The anterior side, which was the point receiving compression, was placed upwards. A certain load was applied on the mid-shaft tibia until fracture occurred. All the specimens were pressed at a displacement of 5 mm/min and a load-deformation curve was plotted simultaneously. Structural properties (including ultimate load and stiffness) were determined from the load–deformation curve. The material properties were determined based on the calculations of the curve with beam bending theory such as flexural modulus.
Culture of rat osteoblastic UMR-106 cells and cell proliferation assay
UMR-106 cells (ATCC no. CRL-1661) were routinely cultured in Dulbecco's modified Eagle's medium supplemented with 10 % fetal bovine serum, penicillin (100 U (60 μg)/ml) and streptomycin (100 μg/ml) at 37°C in a humidified atmosphere of 95 % air and 5 % CO2, as previously described(Reference Xie, Wu and Lai36). At confluence, the medium was changed to phenol red-free Dulbecco's modified Eagle's medium supplemented with 1 % dextran–charcoal-stripped serum fetal bovine serum for 24 h. The cells were then treated with E2 (10 nm), DFTF extracts, isolated compounds or vehicle in the presence or absence of ICI 182780 for 24 h. The cell proliferation rate was measured by a CellTiter 96® AQueous non-Radioactive Cell Proliferation Kit (Promega) with tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulphophenyl)-2H tetrazolium (MTS) and an electron coupling reagent (phenazine methosulphate). Cells were treated with MTS reagent and the absorbance was then recorded in a microplate reader at 490 nm after 2 h of incubation.
Alkaline phosphatase assay
ALP activity was measured directly on the monolayer of cell cultures on a ninety-six-well microplate after treatment with DFTF extract, isolated compounds, E2 (10 nm) or vehicle in the presence or absence of ICI 182780 for 24 h. The assay was carried out by incubating the cells with 10 mm-p-nitrophenylphosphate for 30 min at 37°C. The absorbance of colour change was measured at 405 nm in a microplate reader. A Bradford protein assay was carried out to normalise ALP expression and as ALP activity was expressed U/l per μg protein.
Real-time quantitative PCR analysis
Total RNA was extracted from the treated cells using TRIzol® reagent according to the manufacturer's instructions (Invitrogen). A measure of 2 μg of total RNA was reverse transcribed to complementary DNA using a high-capacity complementary DNA reverse transcription kit (Applied Biosystems). The mRNA of receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG) was determined by quantitative real-time PCR using 7900HT Fast Real-time PCR system (Applied Biosystems). The complementary DNA was amplified with 600 nm of gene-specific primers: for OPG, sense: GACGAGATTGAGAGAACGAG, antisense: GGTGCTTGACTTTCTAGGTG; for RANKL, sense, TCAGGAGTTCCAGCTATGAT, antisense, CCATCAGCTGAAGATAGTCC; and for glyceraldehyde-3-phosphaste dehydrogenase, sense, TACATTTTGCTGATGACTGG, antisense, TGAATGGTAGGAGCTTGACT. The PCR programme was carried out as follows: denaturation 95°C for 20 s, amplification for forty cycles (95°C for 1 s; 53/60°C for 20 s and 72°C for 20 s). Post-PCR dissociation curves were calculated to confirm the specificity of single-target amplification. The amount of complementary DNA was calculated by using a standard curve that was generated from the negative control group (C)(Reference Lau, Chan and Guo42). Expression levels of RANKL and OPG were normalised to the expression of glyceraldehyde-3-phosphaste dehydrogenase (GAPDH), a housekeeping gene. Modulation of osteoclastogenesis was presented as the ratio between the levels of OPG to RANKL mRNA expression.
Statistical analysis
The in vivo data were analysed using one-way ANOVA followed by Tukey's post-test and the in vitro data were analysed by the non-paired Student's t test between the C and each treatment group using the GraphPad PRISM® software package (GraphPad Software, Inc.). Results were expressed as mean values with their standard errors. P values < 0·05 were considered to be significant.
Results
Effects of Drynaria fortunei total flavonoids on ovariectomy-induced changes in biochemical markers and uterus weight in mice
OVX significantly reduced the uterus index and increased the urinary Ca excretion in mice (v. sham, P< 0·05) (Table 1). E2 significantly increased the uterus index and reduced body weight gain, urinary Ca and Dpd level in OVX mice (v. OVX, P< 0·05). DFTF at medium and high doses suppressed body weight gain (v. sham, P< 0·05) and urinary Ca excretion (v. OVX, P< 0·05) in OVX mice. DFTF at the medium dose significantly increased serum Ca (v. sham, P< 0·05) and reduced urinary DPD (v. OVX, P< 0·05) in OVX mice. Unlike E2, DFTF at all doses did not increase the uterus index in OVX mice.
Table 1 Effects of Drynaria fortunei total flavonoids (DFTF) on body weight, uterus weight, and serum and urinary biochemical markers in ovariectomised (OVX) mice* (Mean values with their standard errors, n 8)

Cr, creatinine; Dpd, deoxy-pyridinoline; sham (veh), sham-operated vehicle-treated; OVX (veh), OVX vehicle-treated; E2, 17β-oestradiol (2 μg/g per d); DFTF (low): 0·087 mg/g per d; DFTF (medium), 0·173 mg/g per d; DFTF (high), 0·346 mg/g per d.
* Sham and OVX C57/BL6J mice were subjected to the following treatments for 6 weeks: sham (veh), OVX (veh), E2, DFTF (low), DFTF (medium), DFTF (high). Results were analysed by one-way ANOVA.
† Mean values were significantly differed compared with OVX (veh) (P< 0·05).
‡ Mean values were significantly differed compared with Sham (veh) (P< 0·05).
Effects of Drynaria fortunei total flavonoids on bone properties in ovariectomised mice
OVX weakened bone quality in mice by significantly reducing the BMD, cross-sectional area and SSI at all three measured sites (distal femur, proximal tibia and lumbar spine L1) (v. sham, P< 0·05) (Table 2). E2 significantly improved both total BMD and trabecular BMD of all measured sites in OVX mice (v. OVX, P< 0·05). Total cross-sectional area was found to be improved by E2 only at the lumbar spine L1 site in OVX mice when compared with the sham or OVX group (P< 0·05). The SSI, which reflects torsional bone strength, was significantly increased by E2 at both the distal femur and lumbar spine L1 in OVX mice (v. OVX, P< 0·05). Similarly, DFTF at all doses were able to improve the bone quality in OVX mice (Table 2). DFTF at all doses significantly increased total BMD, trabecular BMD, total cross-sectional area and SSI of distal femur in OVX mice; trabecular cross-sectional area of proximal tibia; as well as total cross-sectional area and SSI of lumbar spine L1 in OVX mice. However, when considering the effectiveness of DFTF on all measured bone sites, DFTF at the medium dose was superior to other doses, as it could statistically increase the level of total and trabecular BMD, total and trabecular cross-sectional area, as well as SSI in OVX mice (v. OVX, P< 0·05).
Table 2 Effects of Drynaria fortunei total flavonoids (DFTF) on bone mineral density (BMD), cross-sectional area, stress–strain index (SSI) of the distal femur, proximal tibia and lumbar spine region L1 in ovariectomised (OVX) mice* (Mean values with their standard errors, n 8)

Sham (veh), sham-operated vehicle-treated; OVX (veh), OVX vehicle-treated; E2, 17β-oestradiol (2 μg/g per d); DFTF (low), 0·087 mg/g per d; DFTF (medium), 0·173 mg/g per d; DFTF (high), 0·346 mg/g per d; ND, not determined.
* Sham and OVX C57/BL6J mice were subjected to the following treatments for 6 weeks: sham (veh), OVX (veh), E2, DFTF (low), DFTF (medium), DFTF (high). Samples were subjected to peripheral quantitative computed tomography measurement. Results were analysed by one-way ANOVA.
† Mean values were significantly different compared with OVX (P< 0·05).
‡ Mean values were significantly different compared with sham (P< 0·05).
Effects of Drynaria fortunei total flavonoids on biomechanical strength in ovariectomised mice
OVX significantly reduced the ultimate load of the tibia diaphysis in mice (v. sham, P< 0·05) (Table 3). Treatment of OVX mice with E2 significantly increased the ultimate load of the tibia diaphysis by 40 % (v. OVX, P>0·001). DFTF at the medium and high doses significantly increased the ultimate load of tibia diaphysis by 26 and 24 % (v. OVX, P>0·05), respectively. DFTF, but not E2, significantly increased the stiffness of the tibia diaphysis in OVX mice, although the stiffness was not significantly reduced by OVX in mice. Significant stimulatory effects on the stiffness of tibia by 83 % (v. OVX, P>0·05) and 100 % (v. OVX, P>0·001) were observed in mice treated with the low and high doses of DFTF treatment, respectively.
Table 3 Effects of Drynaria fortunei total flavonoids (DFTF) on biomechanical bone strength of the tibia diaphysis in ovariectomised (OVX) mice* (Mean values with their standard errors, n 8)
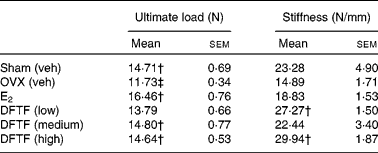
Sham (veh), sham-operated vehicle-treated; OVX (veh), OVX vehicle-treated; E2, 17β-oestradiol (2 μg/g per d); DFTF (low), 0·087 mg/g per d; DFTF (medium), 0·173 mg/g per d; DFTF (high), 0·346 mg/g per d.
* Sham (Veh) and OVX C57/BL6J mice were subjected to treatment with E2, three doses of DFTF (low, medium and high) or its vehicle (OVX (veh)) for 6 weeks. Tibias were subjected to a three-point bending machine. Results were analysed by one-way ANOVA.
† Mean values were significantly different compared with OVX (P< 0·05).
‡ Mean values were significantly different compared with sham (P< 0·05).
Effects of Drynaria fortunei total flavonoids and isolated compounds on cell proliferation in rat osteoblast-like UMR-106 cells
DFTF at 0·02 to 0·2 μg/ml increased cell proliferation in UMR-106 cells (Fig. 2(a), P< 0·001), but a further increase in the concentration reduced cell proliferation. All three isolated compounds stimulated UMR-106 cell proliferation in a dose-dependent manner. As expected, naringin stimulated cell proliferation at 10− 8 to 10− 6m (Table 4). Compound 1 significantly induced cell growth from 10− 12m and increased by 90 % at 10− 8m (Table 4, P< 0·001). Compound 2 also significantly induced cell growth at 10− 12 to 10− 6m, but the maximal fold of induction at 10− 10m was only 27 % (Table 4).

Fig. 2 Effects of Drynaria fortunei total flavonoids (DFTF) on cell proliferation and cell differentiation in rat osteoblast-like UMR-106 cells. DFTF were found to promote (a) cell proliferation and (b) cell differentiation in a dose-dependent manner. UMR-106 cells were treated with 17β-oestradiol (E2, 10 nm), vehicle and DFTF (0·002–20 μg/ml) for 24 h. Cell proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulphophenyl)-2H tetrazolium (MTS) assay, while cell differentiation rate was determined by alkaline phosphatase (ALP) activity, with the results normalised by the amount of total protein. Results were obtained from three independent experiments. Values are means, with their standard errors represented by vertical bars (n 8). *** Mean value was significantly different from that of the control group (C): (P< 0·001).
Table 4 Effects of Drynaria fortunei isolated compounds on cell proliferation and cell differentiation in rat osteoblast-like UMR-106 cells† (Mean values with their standard errors, n 8)

E2, 17β-oestradiol.
Mean values were significantly different compared with the control group: * P< 0·05, ** P< 0·01, *** P< 0·001.
† UMR-106 cells were treated with E2 (10 nm), vehicle, naringin (10− 14 to 10− 6m), compound 1 (10− 14 to 10− 6m) and compound 2 (10− 14 to 10− 6m) for 24 h. Cell proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulphophenyl)-2H tetrazolium assay, while cell differentiation rate was determined by alkaline phosphatase activity with the results being normalised by the amount of total protein. Results were obtained from three independent experiments.
Effects of Drynaria fortunei total flavonoids and isolated compounds on alkaline phosphatase activity in UMR-106 cells
DFTF significantly increased ALP activity in UMR-106 cells at all tested concentrations from 0·002 to 0·2 μg/ml (Fig. 2(b), P< 0·001) and the maximal induction was at 0·02 μg/ml, with an increase of 32 %. Naringin stimulated ALP activity in a dose-dependent manner, with a maximal increase of 12 % at 10− 6m in the UMR-106 cells (Table 4, P< 0·001). Compound 1 was more active at lower concentration, which induced ALP activity by 48 % at 10− 14m (v. C, P< 0·001, Table 4). The fold of induction of ALP activity declined with increasing concentration of compound 1. Compound 2 stimulated ALP activity in a dose-dependent manner and reached the maximum induction by 42 % at 10− 8m (v. C, P< 0·001, Table 4).
Effects of ICI 182780 on the stimulatory effects induced by Drynaria fortunei total flavonoids and isolated compounds in UMR-106 cells
To determine if the stimulatory action of DFTF and isolated compounds in UMR-106 cells were mediated via ER, cells treated with optimal concentrations of respective treatment were co-treated with a specific ER antagonist, ICI 182780. As shown in Fig. 3(a), the stimulatory actions of 10 nm-E2, 0·02 μg/ml DFTF, 10 nm-naringin, 10 nm-compound 1 and 0·1 nm-compound 2 on cell proliferation were completely abolished by co-treatment with ICI 182780. The results suggest that their stimulatory actions on osteoblastic cell proliferation are mediated via ER. Similarly, the stimulatory actions of 10 nm-E2, 0·02 μg/ml DFTF and 10 nm-naringin on ALP activity were abolished in the presence of ICI 182780 in UMR-106 cells (Fig. 3(b)). However, the stimulatory effects of compounds 1 and 2 on ALP activity in UMR-106 cells were not abolished by co-treatment with ICI 182780. The results indicated that the stimulatory actions of E2, DFTF and naringin, but not compounds 1 and 2, on osteoblastic differentiation were ER dependent.

Fig. 3 Effects of ICI 182780 on the stimulatory actions of Drynaria fortunei total flavonoids (DFTF) and isolated compounds on (a) cell proliferation and (b) differentiation in UMR-106 cells. The abolishment of cell proliferation effects was observed in DFTF and its active compounds. However, the abolishment of cell differentiation effects was only observed in DFTF and naringin, but not in compounds 1 and 2. (a) UMR-106 cells were treated with 17β-oestradiol (E2, 10 nm), vehicle, DFTF (0·02 μg/ml), naringin (10 nm), compound 1 (10 nm) and compound 2 (0·1 nm) for 24 h in the presence or absence of ICI 182780 (10 μm). Results were obtained from three independent experiments. Values are means, with their standard errors represented by vertical bars (n 6). Mean values were significantly different compared with the control group (C): * P< 0·05, ** P< 0·01, *** P< 0·001. Mean values were significantly different compared between treatment groups with (□) and without ICI (![]() ): † P< 0·05, †† P< 0·01 and ††† P< 0·001).
): † P< 0·05, †† P< 0·01 and ††† P< 0·001).
Effects of Drynaria fortunei total flavonoids and isolated compounds on osteoprotegrin and receptor activator of NF-κB ligand mRNA expression in UMR-106 cells
A measure of 10 nm of E2, DFTF (0·02 μg/ml), 10 nm-compound 1 and 0·1 nm-compound 2, but not naringin (10 nm), significantly increased OPG mRNA expression in UMR-106 cells (v. C, P< 0·05, Fig. 4(a)). The stimulatory effects on OPG mRNA expression by E2, compounds 1 and 2 were reduced in the presence of the ER antagonist. However, the stimulatory effects of compound 1 on OPG mRNA expression in UMR-106 cells were only suppressed by 18 % in the presence of ICI 182780. Moreover, 10 nm-E2 and 10 nm-naringin could significantly suppress RANKL mRNA expression in UMR-106 cells by 10 and 52 % (v. C, P< 0·05, Fig. 4(b)), respectively. DFTF did not alter RANKL mRNA expression in UMR-106 cells. Compounds 1 and 2 significantly induced RANKL expression by 38 and 58 % (v. C, P< 0·05, Fig. 4(b)), respectively. ICI 182780 itself did not alter the mRNA expression level of RANKL. This implies that the role of ICI is passive and the effects of ICI 182780 on RANKL mRNA expression level could only be demonstrated in the presence of oestrogen-like agents. In contrast, co-treatment with ICI 180780 significantly increased RANKL mRNA expression in cells treated with compound 2 and significantly decreased RANKL mRNA expression in cells treated with compound 1. The results suggest that the actions of compounds 1 and 2 on RANKL mRNA expression might not fully mimic the effects of E2 in UMR-106 cells. The results also indicate that the regulation of RANKL mRNA in UMR-106 cells is complex and appears to be differentially regulated by different compounds as well as during co-treatment with ICI 182780. The ratio of the expression level of OPG to RANKL is used to assess the effects of different treatment on osteoclastogenesis(Reference Xie, Wu and Lai36). As shown in Fig. 4(c), treatment of UMR-106 cells with 10 nm of E2, 0·02 μg/ml DFTF, 10 nm-naringin, 10 nm-compound 1 and 0·1 nm-compound 2 significantly induced the ratio of OPG:RANKL mRNA expression (v. C, P< 0·05), suggesting their inhibitory actions on osteoclastogenesis. Moreover, co-treatment of UMR-106 cells with ICI 182780 significantly reduced the stimulatory effects of all treatment on the ratio of OPG:RANKL mRNA (v. respective group, P< 0·05) (Fig. 4(c)), suggesting that their actions were mediated via ER. It should be noted that the blocking effects by ICI 182780 were almost complete in all treatment groups, except for treatment with DFTF (0·02 μg/ml). The results suggest that the effects of DFTF on osteoclastogenesis might involve other signalling pathways.

Fig. 4 Effects of Drynaria fortunei total flavonoids (DFTF) on osteoprotegerin (OPG) and receptor activator of NF-κB ligand (RANKL) mRNA expression in UMR-106 cells. DFTF increased OPG mRNA expression, while naringin decreased RANKL mRNA expression. Isolated compounds 1 and 2 increased OPG and RANKL mRNA expression. The overall mRNA expressions of OPG and RANKL were all up-regulated by DFTF, naringin, compounds 1 and 2. These effects were blocked by oestrogen receptor (ER) antagonist. UMR-106 cells were treated with 17β-oestradiol (E2, 10 nm), vehicle, DFTF (0·02 μg/ml), naringin (10 nm), compound 1 (10 nm) and compound 2 (0·1 nm) for 24 h in the presence or absence of ICI 182780 (10 μm). The samples were subjected to quantitative real-time PCR analysis of OPG, RANKL and glyceraldehyde-3-phosphaste dehydrogenase (GAPDH) mRNA expression. The (a) OPG mRNA and (b) RANKL mRNA expression as well as (c) the ratio of OPG:RANKL mRNA were normalised by an internal control gene, GAPDH. Results were obtained from two independent experiments. Values are means, with their standard errors represented by vertical bars (n 6). Mean values were significantly different compared with the control group (C): * P< 0·05, *** P< 0·001. Mean values were significantly different compared among treatment groups with (□) and without ICI (![]() ): † P< 0·05, †† P< 0·01.
): † P< 0·05, †† P< 0·01.
Discussion
The present study systematically evaluated the osteoprotective effects and mechanism of actions of DFTF in OVX mice and in rat osteoblast-like UMR-106 cells. In addition, the oestrogenic effects of three active ingredients in DFTF on UMR-106 cells were characterised. The present results clearly showed that DFTF was effective in protecting against OVX-induced loss in bone properties as well as increase in urinary Ca excretion in OVX mice. In addition, the present study showed that DFTF as well as its three active ingredients mimicked oestrogen in stimulating cell proliferation, ALP activity and OPG/RANKL mRNA expression in UMR-106 cells, suggesting that it may exert oestrogen-like effects in promoting osteoblastic functions and inhibiting osteoclastogenesis.
Treatment of OVX mice with DFTF for 6 weeks was shown to improve bone quality at the distal femur, proximal tibia and lumbar spine in OVX mice. Total and trabecular BMD were significantly increased in OVX mice at the distal femur by treatment with DFTF at all doses and at the proximal tibia by treatment with DFTF at the medium and high doses. The positive effects of DFTF on total and trabecular cross-sectional area were dose-dependent as well as site-dependent in OVX mice. Specifically, DFTF at the low and medium doses significantly increased total cross-sectional area at the distal femur, while DFTF at the medium and high doses increased the parameter at the proximal tibia. As for the lumbar spine, DFTF at the medium and high doses increased total BMD and total cross-sectional area, while DFTF at the low dose only increased total cross-sectional area but not total BMD in OVX mice. The effects of DFTF on SSI in OVX mice were also site-dependent. DFTF at all doses significantly increased SSI at the distal femur and lumbar spine. In addition, DFTF also increased biomechanical strength at the tibia diaphysis in OVX mice. The present results are in agreement with those reported by others(Reference Xie, Xu and Zhao43, Reference Zhang, Li and Yang44). Xie et al. (Reference Xie, Xu and Zhao43) reported that 0·108 and 0·216 g/kg per d DFTF significantly restored BMD in the distal femur and lumbar spine of OVX rats by 8–15 and 27–36 %, respectively. Our experiments also revealed similar effects at these sites in OVX mice treated with the medium and high dosages.
The present results showed that oestrogen as well as DFTF at the medium and high dosages (0·173 and 0·346 mg/g per d)) could prevent OVX-induced weight gain in mice. As the animals were pair-fed in the present study, it appeared that DFTF was able to suppress weight gain associated with the changes in energy metabolism in mice during oestrogen deficiency. Similarly, both oestrogen and DFTF at the medium and high doses could reduce OVX-induced urinary Ca loss. Most importantly, the beneficial effects of DFTF on bone, body weight control as well as urinary Ca loss were not associated with an induction of the uterus index in mice. Thus, it appears that the oestrogen-like actions of DFTF in vivo might be distinct from those of oestrogen, and the present results suggest that DFTF exerts oestrogen-like effects in a tissue-specific manner.
The present results showed that DFTF as well as the three isolated active ingredients exerted direct stimulatory effects on osteoblastic cell proliferation and differentiation in rat osteoblast-like UMR-106 cells. The induction of osteoblastic differentiation and mineralisation by a crude extract of DF was reported to be mediated via the regulation of bone morphogenetic protein-2 in pre-osteoblastic MC3T3-E1 cells(Reference Jeong, Lee and Yoon17). In addition, serum from DFTF-treated rats was shown to promote proliferation and differentiation of cultured osteoblasts extracted from newborn Sprague–Dawley rats(Reference Zhang, Li and Yang44). The present results, together with those reported by others(Reference Jeong, Lee and Yoon17, Reference Chen, Ding and Tang23), clearly indicated that DF, its isolated ingredients as well as metabolites exert direct effects on osteoblastic cell proliferation and differentiation.
Naringin, one of the three isolated active ingredients in DFTF, was repeatedly shown in our previous study(Reference Wang, Wang and Zhang26, Reference Pang, Mok and Lai45) as well as others(Reference Wu, Fong and Tsai34, Reference Li, Zeng and Cai35, Reference Wong and Rabie37) to exert direct effects on osteoblastic cell proliferation and differentiation in vitro. Our recent studies(Reference Wang, Wang and Zhang26, Reference Wang, Zhen and Zhang29, Reference Wang, Wong and Yao30) identified bioactive ingredients in DFTF that account for its osteoprotective activities, which include flavonoids(Reference Wang, Wang and Zhang26, Reference Wang, Zhen and Zhang29) and phenylpropanoids(Reference Wang, Wong and Yao30) such as naringenin, kurarinone, kushennol F, xanthogalenol, sophoraflavanone G and ferulic acid glucoside. The present study further investigated the dose–response of oestrogen-like effects of two isolated active compounds, namely, compound 1 and compound 2, in UMR-106 cells. The present results showed that compound 1 was highly potent in stimulating osteoblastic cell proliferation and differentiation, while compound 2 was also highly active in stimulating osteoblastic cell differentiation.
OPG and RANKL are identified as the dominant and final mediators of osteoclastogenesis(Reference Bord, Ireland and Beavan46). The secretion of OPG by osteoblastic cells could block the interaction of RANKL with its functional receptor RANK, expressed on the osteoclastic cell surface, thereby inhibiting osteoclastogenesis. The regulations of osteoclasteogenesis through OPG and RANKL are found to be ER dependent(Reference Chen, Garner and Anderson47). Studies by Chen et al. (Reference Chen, Garner and Anderson47) showed that genistein and daidzein at 10 nm induced OPG mRNA expression in human osteoblastic FOB cell lines in an ER-dependent manner. Similarly, the present study demonstrated that DFTF significantly induced the ratio of OPG:RANKL mRNA expression in osteoblastic cells. Such effects might be due to the presence of naringin as well as compounds 1 and 2 in DFTF, as all three isolated compounds were also effective in inducing the ratio by mimicking the effects of oestrogen. The results also suggest that DFTF not only exerted positive effects on bone formation, but also negatively modulated bone resorption through its action on OPG/RANKL mRNA expression. Such observations were in agreement with those reported by Zhu et al. (Reference Zhu, Wang and Wang48) and Xie et al. (Reference Xie, Xu and Zhao43). Total flavones of DF were shown to up-regulate bone-related gene expressions that are involved in bone formation (such as Smad1 and Smad5)(Reference Zhu, Wang and Wang48), suppress the circulatory level of cytokines involved in inducing bone resorption (TNF-α and IL-6) and stimulate the level of the cytokine (IL-4) associated with the suppression of bone resorption in OVX rats(Reference Xie, Xu and Zhao43).
The present results demonstrated that DFTF can exert positive effects in bone via ER-dependent pathways. The present study demonstrated that the stimulatory effects of DFTF on cell proliferation, differentiation as well as OPG:RANKL ratio in UMR-106 cells could be blocked by co-treatment with ICI 182780, thus providing evidence for the involvement of ER in mediating the actions of DFTF. In addition, the present results showed that the actions of compounds 1 and 2 on osteoblastic proliferation, but not on differentiation, were abolished in the presence of ICI 182780. The results suggest that the actions of compounds 1 and 2 might be distinct from those reported for naringin(Reference Pang, Wang and Mok10). Taken together, the present results indicated that DFTF and its isolated compounds behave as phyto-oestrogens in stimulating ER-dependent actions in bone cells.
The present study clearly demonstrated that DFTF is effective in protecting against OVX-induced bone loss in mice and exerts direct effects in regulating osteoblastic cell growth in an ER-dependent manner. The optimal dose of DFTF for achieving bone-protective effects at three measured sites (distal femur, proximal tibia and lumbar spine) in OVX mice was shown to be 0·173 mg/g per d). Our previous studies have identified the major active ingredients in DFTF that exert direct effects on osteoblastic cells(Reference Wang, Wang and Gao28–Reference Wang, Wong and Yao30). The oestrogen-like anabolic actions of three of the isolated compounds in UMR-106 cells were also demonstrated in the present study. Thus, with the well-defined chemical composition, confirmed efficacy in preclinical studies as well as elucidated mechanism of actions, DFTF appears to be a promising agent for the management of postmenopausal osteoporosis. Future studies will be needed to evaluate the clinical efficacy of DFTF for the treatment of osteoporosis in postmenopausal women.
Acknowledgements
The present work was supported by the National Science Foundation China/Research Grant Council (N_PolyU536/04), HKSAR and the Niche Area Fund (I-BB8N) as well as a Research Studentship (RPC9) from The Hong Kong Polytechnic University. K.-C. W. performed the majority of the laboratory work and contributed to the analysis of data and the writing of the manuscript. W.-Y. P. and S.-K. M. participated in both the cell culture and animal experiments in the present study. X.-L. W. performed the preparation of total flavonoids fraction as well as isolated compounds from D. fortunei total extract. W.-P. L. conduced some of the laboratory work. Hung-Kay Chow supervised the measurement and analysis of biomechanical strength of bone specimen. P.-C. L. played a significant role in the design of the study as well as the analysis of bone specimens using peripheral quantitative computed tomography. X.-S. Y. was a co-investigator and grant holder and played a significant role in the design and performance of the study. M.-S. W. was the principal investigator and grant holder and played a major role in the design and performance of the study, interpretation of the results and the writing of the paper. The authors have no conflicts of interest to declare.










