Horses are trickle feeders possessing a voluminous and elaborate large intestinal tract. They are hindgut fermenters with a complex microbial digestion uniquely adapted to grazing on high-fibre, low-energy fodder. Domestication of the horse has led to this natural pattern of feeding and digestion being disrupted. Consequently, gastrointestinal dysfunction, such as colic, is the single most important cause of mortality among domesticated horses(Reference Clarke, Roberts and Argenzio1–Reference Archer and Proudman7).
The microbial hydrolysis of dietary plant fibre (grass) within the large intestine leads to the release of soluble sugars, which are subsequently fermented to monocarboxylates, notably acetate, propionate and butyrate, referred to as SCFA. A large proportion (60–70 %) of the horse's body energy is provided by SCFA absorbed from the caecum and the colon(Reference Argenzio, Southworth and Stevens8–Reference Bergman10). However, to provide further energy for the demands of work and athletic performance, many horses are fed diets containing readily hydrolysable carbohydrates (hCHO), generally in the form of grain (starch); commonly referred to as concentrate diets. There is strong epidemiological evidence for the feeding of hCHO as a risk factor for equine intestinal dysfunction(Reference Archer and Proudman7). This includes evidence of a dose–response relationship between the amount of hCHO and the risk of disease(Reference Tinker, White and Lessard3, Reference Hillyer, Taylor and Proudman6, Reference Cohen, Gibbs and Woods11). It is suggested that when horses are introduced to diets containing high levels of hCHO (>0·4 % of body weight), a substantial proportion of starch escapes hydrolysis in the small intestine. This reaches the large intestine(Reference Potter, Arnold and Householder12–Reference Dyer, Al-Rammahi and Waterfall14), where it is fermented to products such as lactic acid and CO2. It has been demonstrated that there is a significant increase in lactic acid concentration in the colon of horses fed high-grain diets(Reference Medina, Girard and Jacotot15). This is accompanied by a significant decrease in caecal/colonic pH (from >7·5 to < 6·2)(Reference Medina, Girard and Jacotot15, Reference Julliand, De Frombelle and Drogoul16), leading to perturbation in the microbiota(Reference Clarke, Roberts and Argenzio1, Reference Goodson, Tyznik and Cline17) disposing the horse to intestinal dysfunction. However, little information is available on the precise changes that may occur in these microbial ecosystems and their underlying mechanisms.
We previously investigated the molecular diversity of the microbiota in the large intestine of horses maintained on pasture forage by the analysis of PCR-amplified small subunit (SSU) ribosomal RNA (rRNA) gene sequences(Reference Daly, Stewart and Flint18, Reference Daly and Shirazi-Beechey19). We showed that the majority of the recovered sequences are phylogenetically affiliated to several known groups of anaerobic bacteria, namely the low %G+C Gram-positive bacteria belonging to the phylum Firmicutes, especially the Lachnospiraceae (cluster XIVa of the Clostridiaceae), and the phylum Bacteroidetes, previously known as the Cytophaga–Flexibacter–Bacteroides (CFB) phylum. Other notable members included the Fibrobacter, the Ruminococcaceae and the Bacillus–Lactobacillus–Streptococcus (BLS) group of Firmicutes. These studies revealed that the Lachnospiraceae, the Bacteroidetes and the Spirochaetaceae make up the largest proportion of bacteria in the equine hindgut.
In the present study, we have used oligonucleotide–RNA hybridisation methodology to characterise and compare the abundance of these predominant bacterial communities. We have also measured microbial fermentation products in the colonic contents of three groups of horses, maintained on a grass-only diet or a concentrate diet or affected by simple colonic obstruction and distension (SCOD), a prevalent form of dietary-induced intestinal disease.
Methods
Collection of equine colonic samples
Samples of large-intestinal contents were obtained from six freshly slaughtered horses from the local abattoir (Nantwich, Cheshire, UK) (Group 1). They had been maintained on pasture forage in the mid-Cheshire area with ad libitum access to mature grass comprising mainly of Lolium perenne (average nutritional value 8·5 kJ of digestible energy/g DM). Groups 2 and 3 comprised twelve horses in light work and were maintained on concentrate feed rich in sugar and starch, either euthanised for reasons other than intestinal disease, mainly limb injuries (six horses) or undergoing surgery after diagnosis of SCOD (six horses) at the University of Liverpool (Liverpool, UK) Philip Leverhulme Equine Hospital. SCOD is the clinical diagnosis assigned to a number of non-ischaemic diseases of the equine colon (e.g. pelvic flexure impaction, colonic displacement and nephrosplenic entrapment) that result in severe abdominal pain. Surgical treatment is indicated for the successful resolution of some types of SCOD. The precise dietary formulations and individual additives given to horses were not available. However, the core diet for all concentrate-fed horses consisted of a coarse mix balancer (2–2·5 kg/d; 20–25 kJ digestible energy/g), hay/haylage (6 kg/d; 60 kJ digestible energy/g) and barley (1 kg/d; 13 kJ digestible energy/g). A typical coarse mix composition is micronised cereal, grass/alfalfa pellets, molasses, vegetables and herbs. The time between the last meal and collection of samples was between 12 and 16 h in all cases. The animals sampled for the present study were all client-owned horses undergoing euthanasia or veterinary treatment, and hence outside the scope of the Animals (Scientific Procedures) Act 1986. The samples from client-owned animals undergoing veterinary treatment were obtained with the informed consent of their owners, in accordance with University of Liverpool policies.
The horses were adult (7–12 years old) mares and geldings of various breeds. Euthanasia was either by captive bolt (abattoir) or by the injection of a barbiturate (Philip Leverhulme Equine Hospital). The anaesthetised horses received thiopentane, ketamine and isoflurane. There is no evidence that any of these drugs influence gut microbiota. The horses had not received antibiotics at the time of sampling. Gut contents from the pelvic flexure region of the large intestine were removed promptly (within 10 min), after death or during surgery, placed in cryogenic tubes, frozen in liquid N2 and subsequently stored at − 80°C until use.
Extraction of nucleic acid from gut content samples
Nucleic acid was extracted from samples of gut contents using the method outlined by Lin & Stahl(Reference Lin and Stahl20) and as described previously(Reference Daly, Stewart and Flint18, Reference Daly and Shirazi-Beechey19). Frozen samples were thawed, and approximately 1 g aliquots were transferred to 2 ml screw-cap tubes containing 50 μl of 20 % (w/v) SDS, 500 μl phenol equilibrated with a 0·1 m-citrate buffer (pH 4·3) (Sigma-Aldrich Company Limited, Poole, Dorset, UK) and sterile acid-washed glass beads (0·1 mm diameter). The samples were beaten for 2 min at room temperature using a mini beadbeater (Biospec Corporation, Bartlesville, OK, USA; Stratech Scientific, Newmarket, Suffolk, UK) and transferred to a 60°C water bath for 10 min before an additional 1 min of beating. The aqueous supernatant was extracted again with buffer-equilibrated phenol and then an additional three times with phenol–chloroform–isoamylalcohol (25:24:1). The nucleic acid extract was then treated with RNase-free DNase 1 to remove any contaminating genomic DNA, and extracted again with phenol–chloroform–isoamylalcohol (25:24:1). Total nucleic acid (primarily rRNA) was precipitated by the addition of 3 m-sodium acetate (0·1 volumes) and isopropanol (0·7 volumes). The RNA pellets were washed with 70 % ethanol and resuspended in sterile RNase-free water before final purification with an equal volume of 13 % polyethylene glycol (8000); 1·6 m-NaCl. Purified RNA was then resuspended in sterile RNase-free water and stored at − 80°C. RNA integrity was assessed by agarose gel electrophoresis.
Quantitative oligonucleotide hybridisation
Oligonucleotide probes, targeted to specific bacterial populations of intestinal ecosystems(Reference Daly and Shirazi-Beechey19), were 5′-end labelled with 32P using polynucleotide kinase and [γ-32P]ATP. A tenfold excess of the amount of probe necessary to bind to rRNA applied to the membrane was added to 3 μl of 10 × kinase buffer, 3 μl of 10 mm-spermidine, 1 μl of polynucleotide kinase (New England Biolabs (UK) Limited, Hitchin, Herts, UK), an equimolar amount of end-labelling grade [γ-32P] ATP (Perkin Elmer LAS, Beaconsfield, Bucks, UK), and RNase-free water in a total volume of 30 μl. The reaction was then incubated at 37°C for 30 min. Unincorporated 32P was removed from labelled probes using NICK columns (GE Healthcare UK Limited, Little Chalfont, Bucks, UK). Extracted RNA at 100 μg/ml was denatured by the addition of 2 % (v/v) glutaraldehyde and diluted to a final concentration of 4 μg/ml with RNase-free water containing 1 μg Poly(A)/ml and 0·02 μl of 2 % (w/v) bromophenol blue/ml. A 100 μl volume sample (400 ng) was then applied in triplicate to a positively charged nylon membrane (Hybond N+; GE Healthcare UK Limited) using a slot-blot device under vacuum (Scotlab, Coatbridge, Scotland, UK). Dilution series of known SSU rRNA amounts from appropriate positive controls were included as hybridisation standards on each membrane. The membranes were air dried and rRNA was fixed to the membrane by UV cross-linking (UV Stratalinker 2400; Agilent Technologies, Amsterdam, The Netherlands).
The membranes were prehybridised for 2 h at 40°C in a hybridisation buffer (0·9 m-NaCl, 50 mm-NaH2PO4 (pH 7·0), 5 mm-EDTA, 10 × Denhardt's solution, 0·5 % (w/v) SDS and 0·1 mg Poly(A)/ml) before the addition of 400 μl labelled probe in a volume of 10 ml hybridisation buffer. Hybridisation was performed for 16–18 h at 40°C. After hybridisation, the membranes were washed in 1 × saline-sodium citrate buffer; 1 % (w/v) SDS for 15 min at 40°C, followed by washing twice at the appropriate wash temperature for 30 min as described previously(Reference Daly and Shirazi-Beechey19).
Total SSU rRNA abundance was inferred by the use of a universal hybridisation probe (Univ1390) complementary to all characterised SSU rRNA(Reference Zheng, Alm and Stahl21). Following exposure to X-ray film (Kodak MS; Eastman-Kodak, Henel Hempstead, Herts, UK), hybridisation signals were quantified by scanning densitometry using Phoretix 1D Quantifier v.5.10 (Nonlinear Dynamics, Newcastle-upon-Tyne, UK). For the quantification of SSU rRNA, standard curves were constructed by linear regression from the known SSU rRNA amounts applied to each membrane. The relative population abundance of specific bacterial groups was then calculated as a fraction of the total SSU rRNA abundance as determined by the hybridisation of the universal probe.
Measurement of microbial metabolites
Thawed intestinal contents were centrifuged at 13 000 g (Eppendorf 5415C; Eppendorf UK Limited, Cambridge, UK) and the supernatants were removed for the determination of monocarboxylic acid concentration. The method of Richardson et al. (Reference Richardson, Calder and Stewart22) was used to measure the concentrations of naturally occurring monocarboxylic acids (butyric, propionic and acetic) and other analogues (lactic, valeric and isobutyric acids). Briefly, to 1 ml of gut content liquor, 50 μl of an internal standard (0·1 m-2-ethyl butyric acid) were added. Monocarboxylic acids were then extracted by the addition of concentrated HCl and diethyl ether. The diethyl ether layer extract was derivatised, and the monocarboxylic acids and derivatives were separated by capillary GC and quantified in relation to the internal standard.
Statistical analysis
Data in Figs. 1–3 are presented as medians (interquartiles). The points represent individual animals. The significance of differences was determined by using the Mann–Whitney U test for non-normally distributed data (GraphPad Prism 5; GraphPad Software, Inc. La Jolla, CA, USA). The results were considered significant if P < 0·05. P values were confirmed by one-way repeated-measures ANOVA with Bonferroni's multiple comparison test.

Fig. 1 Increased population abundance of the (a) Lachnospiraceae (cluster XIVa of the Clostridiaceae), (b) Bacteroidetes assemblage (Cytophaga–Flexibacter–Bacteroides) and (c) Bacillus–Lactobacillus–Streptococcus group in concentrate-fed and simple colonic obstruction and distension (SCOD) horses compared with grass-fed horses, as determined by quantitative oligonucleotide-RNA hybridisation. Data are presented as medians and interquartiles, n 6. Points represent individual animals. Significance of differences was determined using the Mann–Whitney U test for non-normally distributed data (GraphPad Prism 5; GraphPad Software, Inc.). Results were considered significant if P < 0·05. P values were confirmed by one-way repeated-measures ANOVA with Bonferroni's multiple comparison test. ** Values were significantly different from that of the grass-fed group (P < 0·01).

Fig. 2 Decreased population abundance of (a) Fibrobacter spp. and (b) Ruminococcaceae (cluster IV of the Clostridiaceae) in concentrate-fed and simple colonic obstruction and distension (SCOD) horses compared with grass-fed horses, as determined by quantitative oligonucleotide-RNA hybridisation. Data are presented as in Fig. 1. ** Values were significantly different from that of the grass-fed group (P < 0·01).

Fig. 3 Population abundance of (a) Veillonellaceae (cluster IX of the Clostridiaceae) and (b) the Spirochaetaceae, as determined by quantitative oligonucleotide-RNA hybridisation, showing no significant differences between grass-fed, concentrate-fed and simple colonic obstruction and distension (SCOD) horses. Data are presented as in Fig. 1. (a) Values were not significantly different for concentrate-fed and SCOD horses from that of the grass-fed group, respectively: * P = 0·82; † P = 0·38. (b) Values were not significantly different for concentrate-fed and SCOD horses from that of the grass-fed group, respectively: * P = 0·82; † P = 0·70.
The data in Table 1 are presented as means and standard errors. The significance of differences was determined using Student's t test (GraphPad Prism 5; GraphPad Software, Inc.). The results were considered significant if P < 0·05. P values were confirmed by one-way repeated-measures ANOVA with Bonferroni's multiple comparison test.
Table 1 Concentration of monocarboxylic acids in equine colonic contents
(Mean values with their standard errors)
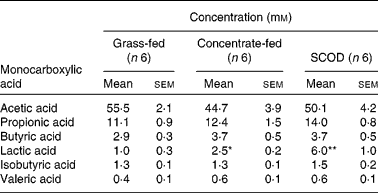
SCOD, simple colonic obstruction and distension.
Mean values were significantly different from that of the grass-fed group and were determined using Student's t test and one-way repeated-measures ANOVA: * P < 0·05; ** P < 0·01.
Results
Alteration in microbiota in response to dietary change and intestinal dysfunction
To characterise the major microbial populations present in the equine colon, we used oligonucleotide–RNA hybridisation to determine the relative population sizes of the specific microbial communities. Bacterial SSU rRNA, extracted from colonic contents of three different groups of horses, was used in conjunction with seven different group-specific oligonucleotide probes to quantify predominant bacterial populations.
The abundance of Lachnospiraceae (cluster XIVa of the Clostridiaceae) and the Bacteroidetes (CFB), targeted by probes EREC482(Reference Franks, Harmsen and Raangs23) and CFB935(Reference Daly and Shirazi-Beechey19), respectively, were significantly greater in concentrate-fed horses and in concentrate-fed horses suffering from SCOD in comparison with horses maintained on grass diet only.
The median population size of the Lachnospiraceae in horses on grass (n 6) was 9·6 % of the total microbiota. However, the population size of this group was higher in all concentrate-fed horses and those with SCOD. The median population sizes were greater, with 25·2 % in the former (n 6; P < 0·01) and 27·6 % in the latter group of horses (n 6; P < 0·01) (Fig. 1(a)).
The median population size of the Bacteroidetes assemblage in grass-fed horses was 13·5 % (n 6). This population was 24·7 % in concentrate-fed horses (n 6; P < 0·01), with all six horses having a significantly higher population. The median population size of the Bacteroidetes in horses with SCOD was 27·7 % (n 6; P < 0·01), with all six horses possessing a significantly higher proportion of Bacteroidetes microbes (Fig. 1(b)).
Similar to the Lachnospiraceae group and the Bacteroidetes assemblage, there was a higher relative population size of the BLS group in both concentrate-fed and SCOD horses. The median population size, as determined by the BLS1295 probe(Reference Daly and Shirazi-Beechey19), was 5·5 % (n 6; P < 0·01) in concentrate-fed horses and 9·2 % in horses with SCOD (n 6; P < 0·01). This compares with the median population size in grass-fed horses of 3·9 % (n 6). All concentrate-fed and SCOD horses contained significantly higher numbers of the BLS group than horses fed on grass only (Fig. 1(c)).
Contrasting effects on other microbial populations were observed in the study groups. The median population size of the Fibrobacter group in grass-fed horses was 2·7 %, as measured by the FIB225 probe(Reference Stahl, Flesher and Mansfield24). However, in concentrate-fed horses and those with SCOD, Fibrobacter comprised 0·4 and 0·5 %, respectively (n 6; P < 0·01 for both groups); all concentrate-fed and SCOD horses contained significantly less Fibrobacter compared with those given grass (Fig. 2(a)).
Using the IV815 probe(Reference Daly and Shirazi-Beechey19), we determined the median population size of the Ruminococcaceae (clostridial cluster IV) to be significantly lower in concentrate-fed horses without (0·3 %; n 6; P < 0·01) and with SCOD (0·2 %; n 6; P < 0·01) compared with grass-fed horses (1·4 %). Again, all concentrate-fed horses and all horses with SCOD had significantly less Ruminococcaceae than those on grass (Fig. 2(b)).
The median population level of Veillonellaceae (probably lactate-utilising microbes) in grass-fed horses was 1·2 % of total, as determined by the IX854 probe(Reference Daly and Shirazi-Beechey19). There was no difference in the relative population size of this microbial group in horses that were either maintained on a concentrate diet or had developed SCOD (median population sizes 1·3 % for both groups) (Fig. 3(a)).
The Spirochaetaceae is a major component of the equine colonic microbiota, comprising up to 15 % of total(Reference Daly and Shirazi-Beechey19). Use of the Spiro1400 probe in the present study showed that the median population size of this group was similar in all three sets of animals (grass 9·9 %; concentrate 10·0 %; SCOD 10·8 %) (Fig. 3(b)).
Changes in microbial metabolites
The mean concentration of lactic acid in the colonic contents of horses fed only on grass was 1·0 mm (n 6), with one horse having no detectable lactic acid. However, the mean steady-state concentration of lactic acid in colonic contents of horses consuming concentrate or having developed SCOD were 2·5 mm (n 6; P < 0·01) and 6·0 mm (n 6; P < 0·01), respectively (Table 1). The concentrations of other monocarboxylic acids (acetic, propionic, butyric, valeric and isobutyric), although variable among individuals, were not significantly different in the intestinal contents of the three groups of horses (Table 1).
Discussion
In the present study, we show that the population sizes of the Lachnospiraceae, the Bacteroidetes (CFB) assemblage and the BLS group are significantly greater in horses fed concentrate and those with the clinical diagnosis of SCOD.
The Lachnospiraceae group is a large and diverse cluster of both fibrolytic and saccharolytic microbes including Clostridium spp., Butyrivibrio spp., Ruminococcus spp. and Eubacterium spp. The Bacteroidetes assemblage in equine colon is comprised mainly of Bacteroides and Prevotella spp., which are predominantly saccharolytic and will readily ferment hCHO(Reference Salyers25). Members of both groups possess the ability to express many different genes involved in fibrolytic/saccharolytic digestion and as such can readily adapt to changes in substrate availability(Reference Schwarz, Zverlov and Bahl26, Reference Backhed, Ley and Sonnenburg27). The enhancement in relative population sizes of these groups is probably a reflection of the increased competitiveness of adaptable members, compared with those that are obligate fibrolytics, in response to the increased amount of hCHO available as substrate.
Members of the BLS, Gram-positive, lactic acid-producing species, favour hCHO as a substrate for fermentation. They do not ferment structural carbohydrates directly but utilise oligosaccharides, released by fibrolytic organisms. They will react rapidly to the availability of more readily fermentable substrates(Reference Nocek28, Reference Owens, Secrist and Hill29)proliferating in a starch-rich environment producing excess amounts of lactic acid and CO2(Reference Goodson, Tyznik and Cline17, Reference Goad, Goad and Nagaraja30). Members of this group, notably Streptococcus bovis, have long been implicated in the aetiology of lactic acidosis both in horses and in ruminants(Reference Nocek28–Reference Russell and Rychlik32).
In contrast, Fibrobacter spp. and members of the Ruminococcaceae (clostridial cluster IV) are obligate fibrolytic, predominantly acid-intolerant bacteria whose growth is greatly suppressed at acidic pH(Reference Russell and Dombrowski33–Reference Miwa, Esaki and Umemori36). The median proportions of these bacterial populations were significantly lower in horses fed concentrate or undergoing surgery for SCOD, suggesting a more acidic colonic pH. Fibrobacter and Ruminococcaceae ferment dietary fibre to SCFA(Reference Dehority, Jung, Buxton, Hatfield and Ralph37). The lower population size of these groups would be expected to result in a reduction in fibre digestion and hence concentrations of intraluminal SCFA. However, our data indicate that the overall concentrations of SCFA remained the same in the intestine of the three study groups of animals. We speculate that this may be due to inhibition of SCFA transport in the intestine of concentrate-fed horses by lactate, a competitive inhibitor of the SCFA transporter, monocarboxylate transporter 1(Reference Ritzhaupt, Ellis and Hosie38–Reference Shirazi-Beechey40).
There were significantly higher concentrations of lactic acid in the colonic contents of concentrate-fed horses and those with SCOD compared with grass-fed horses (2·5- and 6-fold higher concentrations, respectively), correlating with the observed greater population abundance of the BLS group. It has been demonstrated that there is a significant increase in lactic acid concentration in the colon of horses fed high-grain diets(Reference Medina, Girard and Jacotot15). This is accompanied by a marked decrease in caecal/colonic pH (from >7·5 to < 6·2)(Reference Medina, Girard and Jacotot15, Reference Julliand, De Frombelle and Drogoul16). Lactic acid (as opposed to acetic, butyric or propionic acids) is much more likely to cause drastic alterations in colonic pH due to its relatively low pK a of 3·86, thus making it a stronger acid than the other SCFA (the pK a of acetic, butyric and propionic acids are 4·76, 4·82 and 4·87, respectively).
Despite the higher lactic acid concentration, the population size of the Veillonellaceae (lactate utilisers; cluster IX of the Clostridiaceae) was similar (approximately 1 % of total) in each study group of horses. In contrast, representatives of this group can comprise up to 10 % of the total microbial population in the human colon(Reference Duncan, Belenguer and Holtrop41). In addition, one important group of Lachnospiraceae found in human colon (related to Eubacterium hallii and Anaerostipes spp.) has the ability to convert lactate to butyrate(Reference Duncan, Louis and Flint42). Consequently, in humans, most of the lactic acid produced by the fermentation of resistant starch reaching the colon is converted into butyrate and propionate(Reference Stewart, Flint, Bryant, Hobson and Stewart43, Reference Bourriaud, Robins and Martin44). Similarly, in the rumen members of the Veillonellaceae, such as Selenomonas spp., often produce lactate, but many strains can convert lactate into propionate(Reference Belenguer, Duncan and Holtrop45). However, in horses maintained on grass, the median population size of Veillonellaceae was just over 1 % of the total; there was also very little lactic acid present in their colonic contents. The smaller population size of this group in horses may be sufficient when horses are maintained on their natural diet (grass), but an inability to adapt to changes in the colonic environment may contribute to the observed increase in lactic acid.
In order to provide energy for the demands of work and athletic performance, many horses are fed diets containing hCHO, generally in the form of grain (concentrate diet). It is proposed that when they are introduced to diets containing high levels of hCHO (>0·4 % of body weight), a substantial proportion of starch reaches the large intestine(Reference Potter, Arnold and Householder12–Reference Dyer, Al-Rammahi and Waterfall14) causing drastic alteration in microbiota, microbial fermentation products and perturbing caecal and colonic pH(Reference Clarke, Roberts and Argenzio1, Reference Medina, Girard and Jacotot15–Reference Goodson, Tyznik and Cline17) disposing the horse to intestinal dysfunction. Horses have low levels of pancreatic α-amylase, and hence an inability to hydrolyse large amounts of starch, compared with omnivorous species(Reference Roberts46, Reference Kienzle, Radicke and Landes47). It has been shown, in other species, that activity of α-amylase increases in response to dietary hCHO(Reference Brannon48, Reference Swanson, Matthews and Matthews49). There is evidence that horses are also able to enhance the activity of this enzyme in response to increased dietary hCHO, but this is a lengthy process requiring up to 2–3 weeks(Reference Dyer, Al-Rammahi and Waterfall14). Furthermore, the timescale of increased risk of SCOD(Reference Hillyer, Taylor and Proudman6) indicates maximum danger during the 7 d following dietary change (hCHO supplementation), with risk declining to baseline levels by 14 d. This is consistent with the time required for up-regulation of intestinal α-amylase.
Diet has been shown to influence intestinal microbiota resulting in microbial products either beneficial or harmful to the host(Reference Beards, Tuohy and Gibson50, Reference Benus, van der Werf and Welling51). In the present study, we have shown that equine intestinal microbiota are different in horses fed diets varying in hCHO, and that these differences are more exaggerated in a model of dietary-induced intestinal disease. The data demonstrate that differences in diet correlate well with alterations in intestinal microbial ecosystems and production of microbial fermentation products (such as lactic acid), thus creating environments predisposing the host to intestinal diseases.
To further explore associations between dietary change, microbiota and risk of intestinal disease, a case–control study design, where horses of similar age, background and exercise regimen can be managed before dietary intervention, will provide a more direct comparison. A better understanding of molecular mechanisms involved in diet, microbiota and host interactions will allow a more scientifically based approach to designing food formulation, supplementation and management with the ultimate aim of enhancing the health of the species.
Acknowledgements
The financial support of the Horserace Betting Levy Board is gratefully acknowledged. The authors' contributions are as follows: S. P. S.-B. and K. D. designed the study. K. D. performed the study. S. P. S.-B., K. D. and J. D. analysed the data. C. J. P. provided equine colonic samples and dietary/clinical advice. S. H. D. and H. J. F. provided the analytical data on metabolite measurements. S. P. S.-B. and K. D. wrote the manuscript. The authors declare that there is no conflict of interest.






