Adequate provision of protein in diets may play an important role in regulating the expression of genes involved in lipid metabolism( Reference Wu 1 , Reference Duan, Li and Li 2 ). To our knowledge, there are few published studies in this research area. Fat and fatty acids (FA), in either adipose tissue or skeletal muscle, contribute importantly to various aspects of meat quality, and are central to the nutritional value of meat( Reference Li, Duan and Li 3 , Reference Wood, Nute and Richardson 4 ). Early work on meat FA composition focused on adipose tissue, where the bulk of the body's FA was stored. In recent years, there has been more emphasis on muscle because of its significance as a nutritious food( Reference Wu, Fanzo and Miller 5 ).
Skeletal muscle is a complex tissue and has considerable plasticity in its response to dietary intakes of protein and energy. Intramuscular fat (IMF, often termed marbling fat), which is the total lipid within the skeletal muscle, has a role in the tenderness and juiciness of cooked meat( Reference Blanchard, Ellis and Warkup 6 – Reference Li, Li and Yang 10 ). The role of marbling fat is of particular interest in pigs because genetic selection for lean pigs has reduced the level of marbling fat to below 1 % of muscle weight in modern pigs, compared with 2–4 % in US studies in the 1960s. However, in humans and farm animals, excess accumulation of IMF in muscle tissues is associated with conditions such as insulin resistance and type 2 diabetes. Therefore, many intrinsic pathways in both intramuscular adipocytes and myofibres could provide an explanation for the variability of IMF content, such as the balance between lipid anabolic and catabolic pathways, intracellular trafficking of FA and myofibre energetic metabolism in skeletal muscle.
Feeding strategies could change several aspects of meat quality other than eating quality, through the effect on muscle:fat ratio and composition, although the underlying mechanisms have not been elucidated. There is increasing evidence for roles of dietary protein in influencing growth and body composition of aquatic livestock( Reference Mathis, Feidt and Brun-Bellut 11 ) and human subjects( Reference Noakes, Keogh and Foster 12 ). For example, dietary protein:energy ratio is negatively correlated with fat deposition in the carcass. Likewise, when the diet provides an ideal level of crude protein (95 g/kg DM), protein deposition in growing pigs reaches a maximum value of 71 g/d( Reference Barea, Nieto and Aguilera 13 ). The use of low-protein diets to increase marbling fat in pigs has resulted in a higher score for tenderness and juiciness in cooked pork. Several studies have shown that IMF content can be increased by feeding pigs a protein/lysine-deficient diet in the growing or finishing phase. In the study of Teye et al. ( Reference Teye, Sheard and Whittington 14 ), amounts of total lipids in the longissimus dorsi (LD) muscle were 2·8 % when pigs were fed an 18 % crude protein diet, compared with 1·7 % fat in a standard diet containing 20 % protein. The scores for tenderness and juiciness (range 1–8) were markedly increased from 4·2 and 3·9 in the 20 % protein diet to 4·8 and 4·4 in the 18 % protein diet, respectively.
Bama mini-pigs (Sus scrofa domestica), a Chinese indigenous mini-pig breed located in Bama County, Guangxi Province of China, is a useful animal model for studying lipid metabolism( Reference Kawaguchi, Miyoshi and Miura 15 ) and high-quality meat. Landrace pigs, a representative lean genotype, have a fast growing rate and more meat to yield commercial benefits. Although these two breeds show obvious differences in muscle growth and meat quality, how dietary nutrients mediate the effect of breed on lipid metabolism and its underlying mechanism is still unknown. The purpose of the present study was to determine the effects of dietary nutrient levels on fat deposition, FA composition and lipid metabolism in Bama mini-pigs and Landrace pigs, during various phases of their growth.
Materials and methods
Animals, diets and treatment
A total of ninety-six barrows (forty-eight pure-bred Bama mini-pigs (fatty type)( Reference Yang, Fu and Shao 16 ), average initial body weight (BW) 3·38 (sem 0·96) kg, a Chinese local breed; forty-eight Landrace pigs (lean type), average initial BW 7·68 (sem 0·89) kg) were fed from 5 weeks of age to market weight. The experiment was a 2 × 2 factorial arrangement, with two breeds (Bama mini-pigs v. Landrace pigs) and two dietary protein levels (National Research Council diet (NRC diet) v. Chinese conventional diet (GB diet)), giving a total of four treatments (Table 1). Piglets from each breed were randomly assigned to one of the two dietary treatments, with twenty-four piglets in each treatment. The NRC diets were formulated to meet the nutrient requirements recommended by the NRC( 17 ), whereas the GB diets were formulated to meet the recommendations of the Chinese National Feeding Standard for Swine( 18 ), and protein levels of the latter were lower than those of the former (Table 2; online supplementary Table S1). The animals were individually housed in 0·6 m × 1·2 m pens with hard plastic slatted flooring, and had free access to drinking-water and their respective diets( Reference Yin, Ren and Duan 19 ). The dietary phase was based on the physiological stage of pigs.
Table 1 Animals and treatments
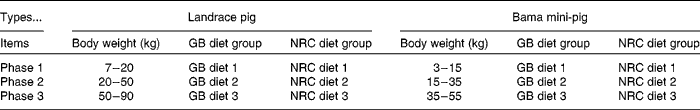
GB diet, Chinese conventional diet; NRC diet, National Research Council diet.
Table 2 Nutrient levels of the experimental diets
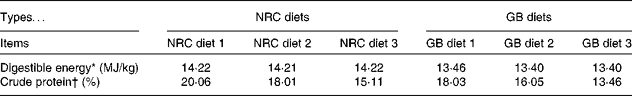
NRC diet, National Research Council diet; GB diet, Chinese conventional diet.
* Digestible energy was the calculated value.
† Crude protein was the measured value.
The experiment was carried out in accordance with the Chinese Guidelines for Animal Welfare and Experimental Protocol, and approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, the Chinese Academy of Sciences( Reference Kong, Wu and Liao 20 , Reference Yin, Liu and Yin 21 ).
Sample collection
BW ranges for the nursery, growing and finishing phases (namely phase 1, phase 2 and phase 3, respectively) were defined as 7–20, 20–50 and 50–90 kg, respectively, for Landrace pigs; and 3–15, 15–35 and 35–55 kg, respectively, for Bama mini-pigs (Table 1). At the end of each phase, eight pigs from each treatment were randomly selected, weighed, bled and killed. In brief, after fasting for 12 h and the measurement of pre-slaughter BW, blood samples were collected into 10 ml centrifuge tubes containing sodium heparin (14·3 United States Pharmacopeia (USP) units/ml), and subsequently centrifuged at 900 g for 10 min at 4°C to recover plasma, and stored at − 80°C until biochemical parameter analysis( Reference Zhou, Kong and Lian 22 – Reference Ren, Yin and Wu 24 ). The pigs were held under general anaesthesia and killed by a jugular vein injection of 4 % sodium pentobarbital solution (40 mg/kg BW). After the head, legs, tail and viscera were removed, the carcass was split longitudinally. Samples of the LD and biceps femoris (BF) muscles on the right-side carcass were collected immediately; visible intermuscular adipose tissue was carefully removed. The samples were snap-frozen in liquid N2, and stored at − 80°C for analysis.( Reference Feng, Zhou and Wu 25 )
Determination of carcass characteristics
Back fat thickness at the tenth rib was measured immediately post-mortem, according to the Chinese Guidelines on Performance Measurement Technology and Regulations for Pigs( 26 ), with the average of measurements at three points: the first rib; last rib; last lumbar vertebra. Each left-side carcass was weighed and then physically dissected into skin, skeletal muscle, fat and bone. Weights of fat and muscle tissues were multiplied by 2 for calculating the percentages of fat and muscle of the whole carcass, respectively.
Intramuscular fat analysis
Analysis of IMF in different muscle tissues was performed in duplicate according to the Association of Official Analytical Chemists method( Reference Cunniff 27 ), using the Soxhlet extraction method( Reference Tan, Yin and Liu 28 ).
Measurements of plasma metabolites
The concentrations of total cholesterol, TAG, LDL and HDL, and activity of lipase in plasma were measured, using a biochemical analytical instrument (Beckman CX4; Beckman Coulter, Inc.) and commercial kits (Sino-German Beijing Leadman Biotech Limited).
Fatty acid composition analysis
The percentages of FA in IMF were determined by Agilent 7890A GC. Briefly, lipids were extracted from the LD and BF muscles using chloroform. Methyl esters of lipids were obtained via saponification with a solution of 2 ml hexane, 40 μl methyl acetate and 100 μl sodium methoxide; the hexane layer was aspirated after vortexing via anhydrous sodium sulphate for the analysis of FA by GC, using a chromatographic column (sp-2560) (100 m × 250 μm × 0·2 μm) (Applied Biosystems). The gas chromatograph programme temperature was as follows: initial column temperature held at 140°C for 15 min; increased at a rate of 3°C/min to 240°C; held for 15 min at 240°C. The injector and detector temperatures were set at 250°C. Hydrogen was used at a flow rate of 30 ml/min, air 400 ml/min, N 40 ml/min and carrier gas 0·8 ml/min. The inlet temperature was 220°C, the split ratio was 10:1 and the injection volume was 1 μl. Individual FA peaks were identified by comparison of their retention times with those of the standards (Sigma Chemicals). Results are expressed as g/100 g of total identified FA( Reference Yin, McEvoy and Souffrant 29 ).
RNA extraction and complementary DNA synthesis
Total RNA was isolated from the LD and BF tissues using the TRIzol reagent (Invitrogen-Life Technologies), and then treated with DNase I (Invitrogen), according to the manufacturer's instructions( Reference Wu, Ruan and Gao 30 ). RNA quality was checked by 1 % agarose gel electrophoresis, and stained with 10 μg/ml of ethidium bromide. RNA was shown to have an optical density (OD)260:OD280 ratio between 1·8 and 2·0. The first-strand complementary DNA was synthesised with oligo(dT)20 and Superscript II reverse transcriptase (Invitrogen), according to the manufacturers' instructions( Reference Wang, Blachier and Fu 31 ).
Quantitative real-time RT-PCR
Primers for the selected genes (see online supplementary Table S2) were designed using the Primer 5.0 software. Real-time RT-PCR was performed using the SYBR Green Detection Kit (TaKaRa), containing MgCl2, deoxy-ribonucleoside triphosphate (dNTP) and HotStar Taq polymerase. An aliquot (2 μl) of a complementary DNA template (corresponding to 25 ng of total RNA) solution was added to a total volume of 10 μl, containing 5 μl SYBR Green mix, 0·2 μl ROX Reference Dye (50 × ), and 0·2 μl each of forward and reverse primers( Reference Liu, Wu and Yin 32 ). After a pre-denaturation programme (10 s at 95°C), forty cycles of amplification were performed (95°C for 10 s and 60°C for 20 s), followed by a melting curve programme (60–99°C with a heating rate of 0·1°C/s and fluorescence measurement); and the fluorescent signal was detected by the ABI Prism 7900HT (Applied Biosystems). A melting curve was generated for each sample at the end of each run to ensure the purity of the amplified products. The amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in each sample was used to normalise the mRNA levels of the selected genes. We calculated the relative expression ratio (R) of mRNA by using the following formula:
where ΔΔC t (sample − control) = (C t gene of interest − C t GAPDH) for the sample − (C t gene of interest − C t GAPDH) for the control.
Real-time RT-PCR efficiencies were determined by the amplification of a dilution series of complementary DNA according to the equation 10( − 1/slope), as described by Bustin et al. ( Reference Bustin, Benes and Garson 33 ), and were consistent between target mRNA and GAPDH. Negative controls, in which complementary DNA was replaced by water, were also used in the experiment( Reference Wang, Shi and Zhang 34 ).
Statistical analysis
Data were analysed by a mixed-effects model using the SAS version 8.2 (SAS Institute, Inc.). Diet, strain and their interactions were included in the statistical model. Effects were considered statistically significant if P< 0·05. Probability values between 0·05 and 0·10 were considered to be trends.
Results
Total lipid and intramuscular fat
Back fat thickness and total fat percentage of carcass, and IMF of the LD and BF muscles in Bama mini-pigs were higher (P< 0·05), whereas muscle percentage of carcass was lower (P< 0·05) than that of Landrace pigs throughout the experiment period (Figs. 1 and 2). The IMF in the BF muscle of Bama mini-pigs fed the GB diet in phases 1 and 2 were greater (P< 0·05) than that of pigs fed the NRC diet, while the IMF in the BF muscle of Bama mini-pigs fed the GB diet in phase 3 was lower (P< 0·05) than that of pigs fed the NRC diet.

Fig. 1 Effects of dietary protein level and strain on backfat thickness (a), fat mass percentage (b) and muscle mass percentage (c) in growing–finishing pigs. ![]() , Landrace pig/Chinese conventional (GB) diet group;
, Landrace pig/Chinese conventional (GB) diet group; ![]() , Landrace pig/National Research Council (NRC) diet group;
, Landrace pig/National Research Council (NRC) diet group; ![]() , Bama mini-pig/GB diet group;
, Bama mini-pig/GB diet group; ![]() , Bama mini-pig/NRC diet group. Values are means (n 8), with their standard errors represented by vertical bars. Effects were considered statistically significant if P< 0·05. S × D, strain × diet interaction.
, Bama mini-pig/NRC diet group. Values are means (n 8), with their standard errors represented by vertical bars. Effects were considered statistically significant if P< 0·05. S × D, strain × diet interaction.

Fig. 2 Effects of dietary protein levels and strains on intramuscular fat (IMF) in the longissimus dorsi (a) and biceps femoris (b) muscles of growing–finishing pigs. ![]() , Landrace pig/Chinese conventional (GB) diet group;
, Landrace pig/Chinese conventional (GB) diet group; ![]() , Landrace pig/National Research Council (NRC) diet group;
, Landrace pig/National Research Council (NRC) diet group; ![]() , Bama mini-pig/GB diet group;
, Bama mini-pig/GB diet group; ![]() , Bama mini-pig/NRC diet group. Values are means (n 8), with their standard errors represented by vertical bars. Effects were considered statistically significant if P< 0·05. S × D, strain × diet interaction.
, Bama mini-pig/NRC diet group. Values are means (n 8), with their standard errors represented by vertical bars. Effects were considered statistically significant if P< 0·05. S × D, strain × diet interaction.
Lipid-related substances in plasma
There was no breed × diet interaction (P>0·05) for plasma concentrations of total cholesterol, TAG, LDL, HDL, and the activity of lipase (Table 3). Plasma LDL concentration and lipase activity of Bama mini-pigs were higher (P< 0·05) than those of Landrace pigs in phase 1, while they showed no difference in phase 2 or phase 3. Landrace pigs fed the GB diet had higher (P< 0·05) TAG concentration in phase 1, and higher (P< 0·05) concentrations of total cholesterol and TAG in phase 2 than those fed the NRC diet.
Table 3 Effects of dietary protein levels and strains on the plasma biochemical parameters in growing–finishing pigs (Mean values with their standard errors, n 8)

GB diet, Chinese conventional diet; NRC, National Research Council diet; LPS, lipase.
* 1 U is defined as the amount of the enzyme that produces a certain amount of enzymatic activity that yields the maximal substrate conversion rate.
Fatty acid composition
As shown in Tables 4 and 5, breed type affected (P< 0·05) percentages of FA, especially PUFA, in the LD and BF muscles, as well as the ratio of PUFA:SFA. For the LD muscle (Table 4), Landrace pigs had a higher PUFA percentage in phases 2 and 3, and a higher PUFA:SFA ratio in phase 2 than Bama mini-pigs. For the BF muscle (Table 5), Bama mini-pigs had a higher (P< 0·05) percentage of MUFA, a lower percentage of PUFA, a lower PUFA:SFA ratio in phase 1, as well as a higher percentage of SFA and a lower PUFA percentage in phase 3, when compared with Landrace pigs. The PUFA percentage of Landrace pigs fed the NRC diet was higher (P< 0·05) than that of pigs fed the GB diet, which resulted in an increase in the PUFA:SFA ratio, while its MUFA percentage was lower (P< 0·05) than those fed the GB diet.
Table 4 Fatty acid percentage in the longissimus dorsi muscle of growing–finishing pigs (Mean values with their standard errors, n 8)

GB diet, Chinese conventional diet; NRC, National Research Council diet.
* SFA = 14 : 0+16 : 0+18 : 0+20 : 0.
† MUFA = 16 : 1+18 : 1.
‡ PUFA = 18 : 2+18 : 3+20 : 4+20 : 5+22 : 6.
Table 5 Fatty acid percentage in the biceps femoris muscle of growing–finishing pigs (Mean values with their standard errors, n 8)

GB diet, Chinese conventional diet; NRC, National Research Council diet.
* SFA = 14 : 0+16 : 0+18 : 0+20 : 0.
† MUFA = 16 : 1+18 : 1.
‡ PUFA = 18 : 2+18 : 3+20 : 4+20 : 5+22 : 6.
Expression of fat metabolic-related genes in muscles
As shown in Figs. 3 and 4, Bama mini-pigs had higher (P< 0·05) mRNA levels of acetyl-CoA carboxylase α (ACCα) and PPARγ coactivator-1α (PGC-1α) in the LD muscle in phase 3, and of ACCα, lipoprotein lipase (LPL) and fatty acid transport protein 1 (FATP-1) in the BF muscle in phase 1, compared with Landrace pigs. Landrace pigs fed the NRC diet had greater (P< 0·05) mRNA levels of hormone-sensitive lipase (HSL) and FATP-1 in the LD muscle in phase 2, higher (P< 0·05) mRNA levels of ACCα, HSL and fatty acid-binding protein 4 (FABP-4) in the BF muscle in phase 2, and lower (P< 0·05) mRNA levels of fatty acid synthase (FAS) in the BF muscle in phase 3 than pigs fed the GB diet. Compared with the effects of the NRC diet, Bama mini-pigs fed the GB diet had higher (P< 0·05) mRNA levels of FAS in phase 1 and lower (P< 0·05) mRNA levels of FAS and LPL in phase 3 in the LD muscle, and higher (P< 0·05) mRNA levels of HSL in phase 1, lower (P< 0·05) mRNA levels of FAS in phase 1, and lower (P< 0·05) mRNA levels of ACCα and PGC-1α in phase 3 in the BF muscle.

Fig. 3 Relative mRNA levels of key regulatory enzymes for fat metabolic-related genes in the longissimus dorsi (a) and biceps femoris (b) muscles of growing–finishing pigs. The mRNA expression levels of acetyl-CoA carboxylase α (ACCα), fatty acid synthase (FAS), hormone sensitive lipase (HSL), and lipoprotein lipase (LPL) were normalised using glyceraldehyde-3-phosphate dehydrogenase as an internal control. ![]() , Landrace pig/Chinese conventional (GB) diet group;
, Landrace pig/Chinese conventional (GB) diet group; ![]() , Landrace pig/National Research Council (NRC) diet group;
, Landrace pig/National Research Council (NRC) diet group; ![]() , Bama mini-pig/GB diet group;
, Bama mini-pig/GB diet group; ![]() , Bama mini-pig/NRC diet group. Values are means (n 8), with their standard errors represented by vertical bars. Effects were considered statistically significant if P< 0·05. S × D, strain × diet interaction.
, Bama mini-pig/NRC diet group. Values are means (n 8), with their standard errors represented by vertical bars. Effects were considered statistically significant if P< 0·05. S × D, strain × diet interaction.

Fig. 4 Relative mRNA levels of fatty acid transport proteins for fat metabolic-related genes in the longissimus dorsi (a) and biceps femoris (b) muscles of growing–finishing pigs. The mRNA expression levels of CCAAT/enhancer-binding protein α (C/EBPα), PPARγ, PPARγ coactivator-1 (PGC-1α), fatty acid transport protein 1 (FATP-1), and fatty acid-binding protein 4 (FABP-4) were normalised using glyceraldehyde-3-phosphate dehydrogenase as an internal control. ![]() , Landrace pig/Chinese conventional (GB) diet group;
, Landrace pig/Chinese conventional (GB) diet group; ![]() , Landrace pig/National Research Council (NRC) diet group;
, Landrace pig/National Research Council (NRC) diet group; ![]() , Bama mini-pig/GB diet group;
, Bama mini-pig/GB diet group; ![]() , Bama mini-pig/NRC diet group. Values are means (n 8), with their standard errors represented by vertical bars. Effects were considered statistically significant if P< 0·05. S × D, strain × diet interaction.
, Bama mini-pig/NRC diet group. Values are means (n 8), with their standard errors represented by vertical bars. Effects were considered statistically significant if P< 0·05. S × D, strain × diet interaction.
Discussion
The results of the present study indicated important effects of breed type on back fat thickness, total fat percentage of carcass, and IMF of the LD and BF muscles. Landrace pigs had less fat and lower IMF than Bama mini-pigs. These findings were, indeed, expected since the aims of genetic selection in pigs, for many years, have been to increase protein growth rate and reduce fat content. Fast-growing genotypes, with a high capacity for muscle protein accretion, such as Landrace pigs, can utilise high-protein and high-energy diets without accumulating excessive amounts of fat (data not shown; YY Liu, XF Kong, FN Li and YL Lin, unpublished results). Moreover, Landrace pigs fed the GB diets containing low levels of energy and protein exerted a higher muscle mass percentage, and a lower fat percentage in the growing phase (Fig. 1). Additionally, the IMF content of Bama mini-pigs was high (5·85 %) and beyond the desired percentage (Fig. 2). This suggested that the proper reduction in the dietary levels of energy and protein would be good for pig production.
Changes in blood total cholesterol and TAG contents can reflect dynamic lipid absorption and nutritional status in animals. Previous research has shown that the total cholesterol level is positively associated with body fat deposition( Reference Sink, Wilson and McCarthy 35 ) and the incidence of CHD. LDL is the main carrier of total cholesterol, which is an important index to reflect excessive deposition of total cholesterol in the body. As reviewed by Anderson & Konz( Reference Anderson and Konz 36 ), a 1 % increase in either total or LDL-cholesterol increases the risk for CHD by 2–3 %, whereas the HDL content can be representative of total cholesterol clearance. In the present study, plasma levels of LDL and lipase in Bama mini-pigs were higher than those in Landrace pigs in the nursery phase, implying that the rate of fat synthesis was much greater than that of fat catabolism in Bama mini-pigs. In contrast, total cholesterol and TAG in Landrace pigs fed the NRC diet were lesser than those in pigs fed the GB diet, which reflects, to some extent, a better health condition of Landrace pigs than of Bama mini-pigs. The present results support the notion that both genes and dietary protein intake affect lipid metabolism in animals.
FA in the pig's carcass comes from two sources. Some FA, such as SFA and MUFA, are synthesised by the pig, whereas others, especially PUFA, are obtained from the diet and deposited unchanged in the tissues. In the present study, the percentages of SFA and MUFA in Bama mini-pigs were higher than those in Landrace pigs, indicating that Bama mini-pigs had a better ability of FA synthesis. FA composition influences the oxidative stability of muscle, which, in turn, affects flavour and odour of the muscle. A growing body of evidence shows( Reference Wood, Enser and Fisher 8 ) that there is a high correlation between FA composition and pork tenderness, juiciness and flavour. Unsaturated FA is not only an important precursor substance of meat aroma, but also has a close relationship with the nutritional value of meat. There is a negative correlation between PUFA and meat flavour or overall acceptability, and a positive correlation between MUFA and meat flavour or overall acceptability( Reference Cameron, Enser and Nute 37 ). However, PUFA content is related to the nutritional value of meat( Reference Duan, Li and Li 2 ). The proportion of linoleic acid (18 : 2n-6) in the muscle declines as fat deposition proceeds, which is an index of fatness( Reference Wood, Enser and Fisher 8 ). In the present study, the 18 : 2 content of Bama mini-pigs was lower than that of Landrace pigs, especially during the growing and finishing phases (data not shown), indicating that Bama mini-pigs had greater fat deposition. These findings are in accordance with the carcass characteristic data from the present study. Landrace pigs fed the NRC diet exhibited higher PUFA content, compared with those fed the GB diet. We suggest that the high levels of energy and protein in the diet can enhance the synthetic capacity of PUFA in swine.
Lipogenesis and lipolysis are major factors affecting adipose accumulation in tissues. At present, a few candidate genes associated with meat quality have been identified, including genes related to IMF, such as PPARγ and PGC-1α. The ACC and FAS are key regulatory enzymes in FA synthesis( Reference Munday 38 , Reference Chen, Yang and Tong 39 ). ACCα (the major form of ACC) is a rate-limiting enzyme in long-chain FA de novo synthesis( Reference Tan, Yin and Liu 40 ). ACC catalyses acetyl-CoA to malonyl-CoA in the first step reaction of FA synthesis, and FAS catalyses acetyl-CoA and malonyl-CoA to long-chain FA( Reference Loftus, Jaworsky and Frehywot 41 ). Therefore, ACCα and FAS can modulate fat deposition in animals by regulating FA synthesis. Partitioning of FA between TAG storage in the white adipose tissue and oxidation in the skeletal muscle can be modulated by LPL, which functions as a ‘metabolic gatekeeper’( Reference Zechner 42 ). LPL, as a rate-limiting enzyme in the metabolism of TAG, catalyses the hydrolysis of TAG in chylomicrons and VLDL, to FA. TAG hydrolysis in adipose tissue is also catalysed by HSL( Reference Zou and Shao 43 ). In the present study, the mRNA levels of both ACCα and LPL were up-regulated substantially in Bama mini-pigs than those in Landrace pigs. This may contribute to a superior capability of FA synthesis in the white adipose tissue of Bama mini-pigs. Furthermore, the expression of ACCα and PGC-1α was enhanced in the BF muscle of Bama mini-pigs fed the NRC diet, compared with those fed the GB diet in phase 3, which resulted in higher IMF deposition in Bama mini-pigs. Interestingly, Bama mini-pigs fed the GB diet had a higher mRNA level of HSL, but a lower mRNA level of FAS in the BF muscle during phase 1, when compared with those fed the NRC diet, but no difference was detected in other growth phases. We surmise that, during the initial growing period, the anabolism and catabolism of lipids were both stimulated in the BF muscle of Bama mini-pigs fed the NRC diet, containing high levels of energy and protein, although the IMF content of Bama mini-pigs fed the GB diet was higher than that of those fed the NRC diet.
Overall, three groups of putative FATP have been identified and characterised( Reference Binnert, Koistinen and Martin 44 ): (1) FA translocase; (2) plasma membrane FABP; (3) the family of FATP. The transport of FA into many tissues occurs via a protein-mediated mechanism( Reference Bonen, Chabowski and Luiken 45 ), and FATP, which is expressed in a tissue-specific pattern, plays an important role in FA transport( Reference DiRusso, Li and Darwis 46 ). Fasting increases FATP-1 expression in the mouse adipose tissue, whereas refeeding restores the basal level of this gene( Reference Man, Hui and Schaffer 47 ), indicating that the nutritional status modulates FATP-1 expression in vivo. In the present study, the mRNA level of FATP-1 was up-regulated markedly in Bama mini-pigs than in Landrace pigs during the nursery phase, which might contribute to its stronger elevation in FA uptake and lipogenesis in the tissue at an early stage. Interestingly, we found that Landrace pigs fed the NRC diet had higher mRNA levels of FATP-1 and FABP-4 in the skeletal muscle during the growing period, demonstrating a higher capacity for the transport of long-chain FA (Table 5). This may help explain the balance between lipid anabolic and catabolic pathways in myofibres, as well as energy metabolism in intramuscular adipocytes.
In conclusion, the present study showed that dietary levels of protein regulated lipid anabolism and catabolism via modulating the mRNA levels of key regulatory enzymes and FATP in different muscle tissues of Bama mini-pigs (fatty genotype) and Landrace pigs (lean genotype). The effects were also reflected in the plasma concentrations of metabolites and carcass characteristics. Proper reduction of the dietary levels of protein and energy may be beneficial for pig production. These results suggest that dietary protein intake affects the expression of genes involved in lipid metabolism in the porcine skeletal muscle in a genotype-dependent manner. It provides not only a molecular mechanism, but also has important implications for developing novel dietary strategies in pig production.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514004310
Acknowledgements
The present study was jointly supported by grants from the National Basic Research Program of China (no. 2012CB124704 and 2013CB127305), the National Nature Science Foundation of China (31372325), the K.C. Wong Education Foundation (Hong Kong), and the Texas A&M AgriLife Research. The funding agencies had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: X. K. and Y. Y. conceived and designed the study; Y. L. and F. L. conducted the animal trial, analysed and interpreted the data and wrote the paper; B. T. and G. W. revised the manuscript; L. H. and Y. L. performed the chemical analyses; J. D., Y. L. and M. G. assisted with tissue collection. All authors read and approved the final version of the manuscript.
The authors declare that there is no conflict of interest.












