Introduction
Synaptotagmins are a family of Ca2+-sensing proteins that facilitate synaptic exocytosis. Synaptotagmins -1, -2, and -9 serve as Ca2+ sensors that trigger fast synchronous release in CNS neurons (Geppert et al., Reference Geppert, Goda, Hammer, Li, Rosahl, Stevens and Sudhof1994; Pang et al., Reference Pang, Melicoff, Padgett, Liu, Teich, Dickey, Lin, Adachi and Sudhof2006; Xu et al., Reference Xu, Mashimo and Sudhof2007). The less-studied synaptotagmin-9 (Syt9) shares considerable sequence homology with Syt1 and Syt2 (Rickman et al., Reference Rickman, Craxton, Osborne and Davletov2004). Due to a nomenclature issue in the earlier literature, Syt9 was sometimes referred to as Syt5 and vice versa (Fukuda & Sagi-Eisenberg, 2008), so one must use care when reviewing the literature. For these experiments, we used Syt9 NCBI gene ID 60510 (491 amino acids). In the brain, Syt9 is expressed primarily in the hypothalamus, nucleus accumbens, striatum, and olfactory bulb (Xu et al., Reference Xu, Mashimo and Sudhof2007; Mittelsteadt et al., Reference Mittelsteadt, Seifert, Alvarez-Baron, Steinhauser, Becker and Schoch2009) (http://mouse.brain-map.org). Syt9 has been found to mediate fast, synchronous release in these areas, albeit with slower kinetics than Syt1 or Syt2 (Xu et al., Reference Xu, Mashimo and Sudhof2007; Kochubey et al., Reference Kochubey, Babai and Schneggenburger2016), along with a lower Ca2+ affinity (Hui et al., Reference Hui, Bai, Wang, Sugimori, Llinas and Chapman2005). Eliminating Syt9 from striatal neurons abolished fast synchronous release of γ-aminobutyric acid (GABA) (Xu et al., Reference Xu, Mashimo and Sudhof2007) but a different study in cultured neurons (hippocampal, cortical, and striatal) found that deletion of Syt9 had no effect on evoked inhibitory currents (Seibert et al., Reference Seibert, Evans, Stanley, Wu, Briguglio and Chapman2023). However, this same study showed that in Syt1 KO neurons, overexpressing Syt9 to levels matching Syt1 expression (~25-fold) was able to rescue loss of synchronous release. Elimination of Syt9 in cultured striatal neurons also resulted in lower spontaneous release of GABA (Seibert et al., Reference Seibert, Evans, Stanley, Wu, Briguglio and Chapman2023). Syt9 is present on both small synaptic and large dense core vesicles (DCVs) of the mouse brain (Fukuda, Reference Fukuda2006), and has been found to regulate catecholamine secretion (along with Syt1) from DCVs in cultured PC12 cells (Fukuda et al., Reference Fukuda, Kowalchyk, Zhang, Martin and Mikoshiba2002; Lynch & Martin, Reference Lynch and Martin2007). In the gonadotrophs of female mice, Syt9 has been shown to regulate the release of follicle-stimulating hormone (FSH) from DCVs (Roper et al., Reference Roper, Briguglio, Evans, Jackson and Chapman2015).
Nothing is known about possible roles of Syt9 in retina. Consistent with evidence for Syt9 genes in zebrafish retina (Henry et al., Reference Henry, Joselevitch, Matthews and Wollmuth2022), we show evidence for Syt9 in multiple retinal neurons. Selective elimination of Syt9 from rods caused a reduction in scotopic b-waves and oscillatory potentials (OPs), while global elimination of Syt9 increased b-waves, suggesting actions at multiple sites in the retina.
Materials and methods
Mice
We used control C57Bl6J and mutant mice aged 4–8 weeks for these experiments. Creation of HRGP-Cre and Rho-iCre mice have been described previously (Le et al., Reference Le, Ash, Al-Ubaidi, Chen, Ma and Anderson2004; Li et al., Reference Li, Chen, Sauve, McCandless, Chen and Chen2005). Creation of Syt9fl mice are described elsewhere (Quadros et al., Reference Quadros, Miura, Harms, Akatsuka, Sato, Aida, Redder, Richardson, Inagaki and Sakai2017). Syt9fl mice were originally created using C57Bl6N mice and then back-crossed to C7Bl6J mice. Yun Le (University of Oklahoma) generously provided HRGP-Cre mice. Rho-iCre mice were obtained from Jackson Laboratories, Bar Harbor, ME, USA (B6.Cg-Pde6b+Tg(Rho-iCre)1Ck/Boc; RRID: 015850). Rho-iCre and HRGP-Cre mice selectively express cre-recombinase in rods and cones, respectively (Le et al., Reference Le, Ash, Al-Ubaidi, Chen, Ma and Anderson2004; Li et al., Reference Li, Chen, Sauve, McCandless, Chen and Chen2005; Jin et al., Reference Jin, Zhang, Keung, Youn, Ishibashi, Tian, Marshak, Solessio, Umino and Fahrenfort2020). CMV-Cre mice obtained from Jackson Laboratories (B6.C-Tg(CMV-cre)1Cgn/J) produce ubiquitous expression of cre-recombinase under control of a minimal human cytomegalovirus promoter.
Euthanasia was conducted in accordance with AVMA Guidelines for the Euthanasia of Animals by CO2 asphyxiation followed by cervical dislocation. Animal care and handling protocols were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee.
RNAscope
Eyes were fixed in either 4% paraformaldehyde (PFA) or 10% neutral buffered formalin (NBF) for 48 h, transferred to 70% ethanol, and processed for paraffin embedding (UNMC Tissue Sciences Facility). The following steps were performed using the RNAscope™ 2.5 HD Reagent Kit (RED) along with the Quick Guide for FFPE Tissues (Advanced Cell Diagnostics, Newark, CA, USA; Cat No: 322350). Five-micron thick retina slices were baked at 60ºC, followed by deparaffinization in a series of xylene and 100% ethanol baths at room temperature. Slides were set to air dry for 5 min to prepare for pretreatment. Pretreatment of slides included direct application of hydrogen peroxide to tissue sections for 10 min followed by consecutive distilled water washes. Heat-induced antigen retrieval was completed by submerging sections into Target Retrieval solution at 95–100ºC, sustained by hot water bath. Prior to in situ hybridization, tissue was digested using Protease Plus enzyme solution at 40°C in a humidity chamber.
In situ hybridization for Syt9 RNA was performed using probes targeting Syt9 (Advanced Cell Diagnostics, Newark, CA, USA; Lot #:20293B). Probe Mm-Syt 9 (Cat No. 845291 from ACD Bio) targets the base pair region 636–1595 of the Syt9 transcript (NCBI accession number NM_021889.4). This target region includes a portion of exon 2; the entirety of exons 3, 4, and 5; and a portion of exon 6. Target amplification and color development was done using the 2.5 HD Red Detection sub-kit. Visualization was achieved utilizing Fast Red dye and sections were counterstained with Hematoxylin QS (Vector Laboratories, Burlingame, CA, USA). Images were captured using SPOT Basic software (SPOT Imaging, Diagnostic Instruments Inc., Foxboro, MA) on a Spot Idea color camera (Diagnostics Instruments, Model 28.2) and Leitz Diaplan upright microscope with 40X objective.
Electroretinography
Electroretinograms (ERGs) were recorded in vivo using a UTAS Sunburst ganzfeld illuminator (LKC, Gaithersburg, MD, USA, LKC-UTAS-SB). Mice were dark-adapted for ∼12 h prior to experiments and then anaesthetized via intra-peritoneal injection with a ketamine/xylazine drug cocktail (100 mg/kg ketamine, 10 mg/kg xylazine). Core temperature of the mouse was maintained at 37°C with a heat pad. Tropicamide and proparacaine ophthalmic solution (0.5%) were administered topically to the left eye before the mouse was secured to the platform and a silver/silver chloride wire ring recording electrode was centered on the left cornea. Subcutaneous ground and reference electrodes were placed at the base of the tail and under the scalp, respectively. ERG a-waves provide a measure of photoreceptor responses and were measured from baseline to the bottom of the downward going negative potential. ERG b-waves reflect responses of second-order ON-type bipolar cells and were measured from the trough of the initial negative deflection of the a-wave to the peak of the positive-going b-wave. Measurements in dark-adapted (scotopic) conditions involved flashes of increasing intensity: 51 dB, −45 dB, −39 dB, −33 dB, −27 dB, −21 dB, −15 dB, −9 dB, −3 dB, and +5 dB. The intensity at 0 dB = 2.5 cd·s/m2. Ten flashes were presented at each intensity, separated by 10 s for steps 1–9 and 20 s between flashes at the highest intensity. Light-adapted (photopic) protocols were performed after background adaptation for 10 min with green 530 nm light (40 cd/m2) and conducted with the same background. We tested six intensities (−6, −3, 0, 4, 7, and 13 dB) with 25 flashes at each intensity separated by 3 s. OPs were extracted from flash ERGs by bandpass filtering the entire trace to remove frequency components below 70 Hz and above 280 Hz using an 8-pole Bessel filter in Clampfit (Axon Instruments, Molecular Devices).
Whole cell recordings
Whole cell recordings from rods were performed using a flatmount preparation of isolated retina. After enucleation, each eye was immediately placed in Ames’ medium (US Biological; RRID:SCR_013653) bubbled with 95% O2/5% CO2. The cornea was punctured with a scalpel and the anterior segment removed. The retina was isolated after cutting optic nerve attachments. After making four fine cuts at opposite poles, the retina was flattened onto a glass slide in the perfusion chamber with photoreceptors facing up. The retina was anchored in place with a brain slice harp (Warner Instruments, cat. no. 64–0250). The perfusion chamber was placed on an upright fixed-stage microscope (Nikon E600FN) equipped with a 60x water-immersion, long-working distance objective (1.0 NA). Flatmount preparations were superfused with room temperature Ames solution bubbled with 95%/5% CO2 at ~1 mL/min. Outer segments were removed by gentle suction using a patch pipette to expose inner segments for recording.
Patch recording electrodes were pulled on a Narishige (Amityville, NY, USA) PP-830 vertical puller using borosilicate glass pipettes (1.2-mm outer diameter, 0.9-inner diameter with internal filament; World Precision Instruments, Sarasota, FL, USA). Pipettes had tip diameters of 1–2 μm and resistances of 10–15 MΩ. Rod inner segments and cell bodies were identified in flatmount retina and targeted with positive pressure using recording electrodes mounted on Huxley-Wall or motorized micromanipulators (Sutter Instruments). Cones were distinguished from rods by their larger membrane capacitance and much larger Ca2+ currents.
Rod ribbons are surrounded by the glutamate transporter EAAT5 and glutamate reuptake into rods by EAAT5 activates a large, anion conductance (Picaud et al., Reference Picaud, Larsson, Grant, Lecar and Werblin1995; Grant & Werblin, Reference Grant and Werblin1996; Arriza et al., Reference Arriza, Eliasof, Kavanaugh and Amara1997; Eliasof et al., Reference Eliasof, Arriza, Leighton, Kavanaugh and Amara1998; Hasegawa et al., Reference Hasegawa, Obara, Tanaka and Tachibana2006; Schneider et al., Reference Schneider, Cordeiro, Machtens, Braams, Rauen and Fahlke2014; Grassmeyer et al., Reference Grassmeyer, Cahill, Hays, Barta, Quadros, Gurumurthy and Thoreson2019; Thoreson & Chhunchha, Reference Thoreson and Chhunchha2023). I A(Glu) is activated during glutamate re-uptake but is thermodynamically uncoupled from the transport process (Machtens et al., Reference Machtens, Kortzak, Lansche, Leinenweber, Kilian, Begemann, Zachariae, Ewers, de Groot and Briones2015). To enhance I A(Glu), Cl− in the patch pipette was replaced with a more permeable anion, thiocyanate (Eliasof & Jahr, Reference Eliasof and Jahr1996). The intracellular pipette solution for these experiments contained (in mM): 120 KSCN, 10 TEA-Cl, 10 HEPES, 1 CaCl2, 1 MgCl2, 0.5 Na-GTP, 5 Mg-ATP, 5 EGTA, 5 phospho-creatine, pH 7.2.
Rod I A(Glu) recordings were performed in whole-cell voltage clamp using an Axopatch 200B amplifier (Molecular Devices) and signals were digitized with a DigiData 1550 (Molecular Devices). Data acquisition and analysis were performed using pClamp 10 software (Molecular Devices). Currents were acquired at 10 kHz and filtered at 2 kHz. Passive membrane resistance was subtracted from I A(Glu) using P/6 subtraction. Membrane capacitance, membrane resistance, and access resistance values for rods using the KSCN solution averaged 3.0 ± 0.4 pF, 3.4 ± 2.0 GΩ, and 55 ± 9.5 MΩ (n = 9), respectively. Voltages were not corrected for a liquid junction potential of 3.9 mV. Chemical reagents were obtained from Sigma-Aldrich unless otherwise indicated.
Statistical analysis
Statistical analysis and data visualization were done using ClampFit 10 and GraphPad Prism 9 software. Roughly, equal numbers of male and female mice were used for these experiments. For ERG measurements, we analyzed the sample for each condition using Dunnett’s multiple comparisons test with one-way ANOVA. For comparing spontaneous release rates, we used nested t-tests to compare samples from cells where multiple measurements were made. Data values in the text are reported as mean ± SD. Errors bars for whole cell measurements show 95% confidence intervals.
Results
Localization of Syt9 mRNA in the retina
We used RNAscope techniques to localize sites of Syt9 mRNA in the retina. As illustrated in Fig. 1A–D, we first studied tissue fixed with 4% PFA. In control C56Bl6J mice, labeling for mRNA (red puncta) could be seen in rod photoreceptors (arrows, Fig. 1A), inner retinal neurons (Fig. 1A, B), and an occasional retinal ganglion cell (Fig. 1C). Rods showing RNAscope labeling for Syt9 are noted by arrows in the magnified section of Fig. 1B. Labeling for Syt9 mRNA in photoreceptor inner segments was eliminated in rod-specific conditional Syt9 knockout mice (RodSyt9CKO, Fig. 1C, D). In retinas fixed with 10% NBF, mRNA labeling was diminished but we still saw labeling in photoreceptors and inner retinal neurons (arrows, Fig. 1F). Labeling was abolished entirely in a whole animal knockout of Syt9 (CMVSyt9) created by crossing floxed Syt9 mice with CMV-Cre mice that express cre-recombinase constitutively (Fig. 1G, H). Retinal thickness measured near the center of fixed retinas did not vary significantly among the three genotypes (WT: 220.4 ± 15.2 μm, SD, n = 5 mice; CMVSyt9, 213.5 ± 15 μm, n = 5 mice; RodSyt9CKO, 221.5 ± 25.4 μm, n = 3 mice; P = 0.735, one-way ANOVA).

Figure 1. Syt9 mRNA visualized using RNAscope techniques (red puncta) was seen in the outer nuclear layer (ONL), inner nuclear layer (INL), and ganglion cell layer (GCL). Panels A–D show tissue fixed with 4% paraformaldehyde (PFA). Panels E–H show tissue fixed with 10% neutral buffered formalin (NBF). Panel A shows a retinal section of control C57Bl6J retina fixed with 4% PFA. Panel B shows a magnified region of the same section. Syt9 labeling (red puncta) in rods is indicated by the arrows in panel B. Panel C and magnified region in panel D show that labeling for Syt9 mRNA was eliminated from rods in rod-specific conditional Syt9 knockout mice (RodSyt9CKO), but remained in inner retinal neurons and ganglion cells. Panel E shows a different control C57Bl6J retina fixed with 10% NBF. Labeling of neurons in the ONL and INL (F; arrows) was also seen with this fixative, but fewer cells were labeled. Labeling was abolished altogether in a whole animal knockout of Syt9 created by crossing floxed Syt9 mice with CMV-Cre mice that express cre-recombinase constitutively (CMVSyt9).
ERG effects of global elimination of Syt9
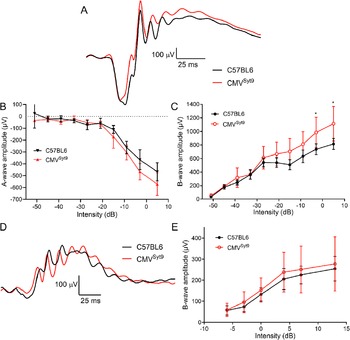
To study the role of Syt9 in synaptic transmission from photoreceptors in shaping bipolar cell light responses, we measured ERGs in vivo from anesthetized mice. Fig. 2A shows representative ERG responses evoked by a high intensity 20 ms flash applied under scotopic conditions to dark-adapted control mice (black trace) and mice in which Syt9 had been globally eliminated (CMVSyt9; red trace). The negative a-wave provides a measure of photoreceptor responses, while the positive b-wave is a measure of second-order ON-type bipolar cell responses. Fig. 2B, C display scotopic a- and b-wave amplitudes, respectively, as a function of increasing flash intensity. Example waveforms for photopic b-waves of light-adapted control (black) and CMVSyt9 (red) mice are shown in Fig. 2D. Fig. 2E plots the amplitude of photopic b-wave responses with increasing light intensity. Global elimination of Syt9 increased scotopic b-waves evoked by high intensity flashes by up to 37% (Fig. 2C), while scotopic a-waves (Fig. 2B) and photopic b-waves (Fig. 2E) were not significantly different.

Figure 2. Global elimination of Syt9 increased scotopic b-wave responses at the highest flash intensities. (A) Representative scotopic ERG waveforms evoked from a high intensity flash (5 dB) for control (n = 6) and CMVSyt9 (n = 7) mice are shown in black and red traces, respectively. (B) Scotopic a-wave amplitude as a function of flash intensity. (C) Plot of scotopic b-wave amplitude as a function of intensity. (D) Example photopic waveforms evoked from a high intensity flash (13 dB). (E) Plot of photopic b-wave amplitude as a function of intensity. *P < 0.01. Error bars show ±SD.
ERG effects of selectively eliminating Syt9 from cones
Fig. 3 displays measurements from ERGs of control mice and ConeSyt9CKO mice in which Syt9 was selectively eliminated from cones. Fig. 3A shows example scotopic ERG waveforms from dark-adapted control and ConeSyt9CKO mice (black and red traces). Scotopic a- and b-wave amplitudes are plotted in Fig. 3B, C, respectively. Eliminating Syt9 from cones did not alter scotopic a- (Fig. 3B) or b-waves (Fig. 3C) significantly. Fig. 4D shows representative photopic ERG waveforms in light-adapted control and ConeSyt9CKO mice. Photopic b-waves from ConeSyt9CKO mice did not differ significantly from control mice (Fig. 3E).

Figure 3. Elimination of Syt9 selectively from cones had no effect on scotopic or photopic ERGs. (A) Representative scotopic ERG waveforms evoked from a high intensity flash (5 dB) for control (n = 6) and ConeSyt9CKO (n = 7) mice are shown in black and red traces, respectively. (B) Scotopic a-wave amplitude as a function of flash intensity. (C) Plot of scotopic b-wave amplitude as a function of intensity. (D) Example photopic waveforms evoked from a high intensity flash (13 dB). (E) Plot of photopic b-wave amplitude as a function of intensity. Error bars show ±SD.

Figure 4. Elimination of Syt9 selectively from rods decreased scotopic and photopic ERG b-waves. (A) Representative scotopic ERG waveforms (5 dB) for control and RodSyt9CKO (n = 4) mice are shown in black and red traces, respectively. (B) Scotopic a-wave amplitude as a function of flash intensity. (C) Plot of scotopic b-wave amplitude as a function of intensity. (D) Example photopic waveforms evoked from a high intensity flash (13 dB). (E) Plot of photopic b-wave amplitude as a function of flash intensity. *P < 0.01. Error bars show ±SD.
ERG effects of selectively eliminating Syt9 from rods
Fig. 4A shows representative scotopic ERGs evoked by a high intensity 20 ms flash applied to dark-adapted control mice and RodSyt9CKO mice that lack Syt9 in rods. Scotopic a- and b-wave amplitudes are plotted in Fig. 4B, C, respectively. Selective elimination of Syt9 from rods in RodSyt9CKO mice reduced scotopic b-waves by up to 26% without significant alterations in the a-wave. Photopic ERG example waveforms are shown in Fig. 4D. Surprisingly, although photopic b-waves were not affected by loss of Syt9 from cones, they were diminished by up to 30% following selective elimination of Syt9 from rods in RodSyt9CKO mice (Fig. 4E).
Oscillatory potentials
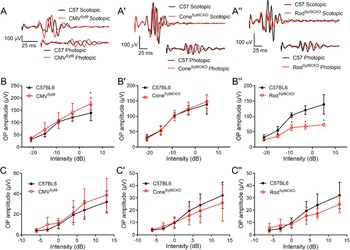
We next looked at effects of Syt9 deletion on ERG OPs that arise from interactions with amacrine cells (Wachtmeister, Reference Wachtmeister1998; Liao et al., Reference Liao, Liu, Milla-Navarro, Villa and Germain2023). Fig. 5 displays measurements of OPs from Syt9 mutant mice under scotopic and photopic conditions. Fig. 5A, A’, A” shows representative OPs from CMVSyt9, ConeSyt9CKO, and RodSyt9CKO mice, respectively. Fig. 5B, B′, B′’ plots scotopic OPs for the five highest intensity flashes of the scotopic ERG protocol. Photopic OPs are plotted in Fig. 5C, C′, C′’. The only significant change in OP amplitudes in CMVSyt9 mice was an increase in scotopic OPs at the brightest flash intensity (Fig. 5B; t-test with Holm–Sidak correction for multiple comparisons, P = 0.00142). Eliminating Syt9 from cones had no effect on OPs under scotopic or photopic conditions (Fig. 5B’, C’). However, eliminating Syt9 from rods reduced OP amplitudes under scotopic conditions, with a 50% reduction at the highest flash intensity (Fig. 5B”; P < 0.00005), but only small changes to OPs under photopic conditions (Fig. 5C”).

Figure 5. Effects of Syt9 elimination from the whole retina on ERG oscillatory potentials (A, A’, A”). Scotopic and photopic OPs extracted from representative ERG waveforms (5 dB and 13 dB, respectively) by bandpass filtering to remove frequencies below 70 Hz and above 280 Hz in CMVSyt9, ConeSyt9CKO, and RodSyt9CKO mice, respectively (B, B’, B”). Scotopic OPs in CMVSyt9, ConeSyt9CKO, and RodSyt9CKO mice, respectively (C, C′, C”). *P < 0.01. Error bars show ±SD.
Whole cell recordings from rods lacking Syt9
Reductions in b-wave amplitude without accompanying changes in a-waves are generally evidence of impaired synaptic release from photoreceptors. Given that selective deletion of Syt9 from rods reduced scotopic b-waves at high intensities (Fig. 4), we recorded directly from rods lacking Syt9 (using CMVSyt9 mice). To assess release, we measured anion currents activated in rods by binding of glutamate to presynaptic EAAT5 glutamate transporters (Grassmeyer et al., Reference Grassmeyer, Cahill, Hays, Barta, Quadros, Gurumurthy and Thoreson2019). The whole cell pipette solution contained SCN− as the principal anion to enhance glutamate transporter anion currents (I A(glu)). We measured inward currents immediately after termination of depolarizing steps from −70 to −10 for 5, 25, and 500 ms. Fig. 6A shows example waveforms of I A(glu) evoked by 25 ms steps in control (top black trace) and Syt9 KO rods (bottom red trace). Passive capacitive and resistive currents were subtracted using P/6 protocols. We measured both amplitude and charge transfer of I A(glu). As illustrated in Fig. 6B, C, we saw no differences in evoked glutamate release between rods lacking Syt9 and a sample of control rods from C57Bl6J mice with either measurement (Fig. 6). We also analyzed spontaneous release in rods with and without Syt9. The downward transients in the example I A(glu) waveforms in Fig. 6D each represent release of a single synaptic vesicle. Spontaneous release was assessed using 30 s trials with rods held at −70 mV. Spontaneous release of glutamate-filled vesicles persisted in the absence of Syt9 with no change in event frequency (Syt9KO: 0.637 ± 0.486 v/s, n = 10 rods; control: 0.641 ± 0.644 v/s, n = 13 rods; nested t-test, P = 0.85).

Figure 6. Whole cell recordings of glutamate transporter anion currents (I A(glu)) revealed no significant differences between control and Syt9 KO rods. (A) Example I A(glu) recordings from control and Syt9 KO rods evoked by 25 ms steps from −70 to −10 mV. Passive capacitive and resistive currents were subtracted using P/6 protocols. (B) I A(glu) amplitude from control (5 ms, n = 34; 25 ms, n = 27; 500 ms, n = 24) and Syt9 KO rods (5 ms, n = 15; 25 ms, n = 16; 500 ms, n = 11). Control data from Mesnard et al. (Reference Mesnard, Barta, Sladek, Zenisek and Thoreson2022). Response amplitude was measured 2 ms after termination of the test step. (C) I A(glu) charge transfer from control (5 ms, n = 37 rods; 25 ms, n = 26; 500 ms, n = 20; n = 15 mice) versus Syt9 KO rods (5 ms, n = 15; 25 ms, n = 16; 500 ms, n = 11; 4 mice). Charge transfer was measured beginning 2 ms after termination of the test step until current recovered to baseline. Baseline was defined as the current level at the end of the 2 s trial. (D) Example recordings of spontaneous I A(glu) release events showing release of individual glutamate-filled synaptic vesicles. Rods were held at −70 mV when measuring spontaneous release. Error bars show 95% confidence intervals.
Discussion
Global Syt9 deletion had different effects on the ERG than selective deletion from rods or cones in mouse retina suggesting involvement of Syt9 at multiple sites in the retina. Global elimination of Syt9 from retina increased the amplitude of scotopic ERG b-wave responses, while deletion from rods alone decreased scotopic ERG b-waves and OPs. Selective deletion of Syt9 from cones had no observable effect.
Consistent with our RNAscope data showing stronger expression of Syt9 mRNA in the inner nuclear layer than in photoreceptors, RNAseq data also show higher Syt9 transcript levels in bipolar cells compared to photoreceptors (Hartl et al., Reference Hartl, Krebs, Juttner, Roska and Schubeler2017; Sarin et al., Reference Sarin, Zuniga-Sanchez, Kurmangaliyev, Cousins, Patel, Hernandez, Zhang, Samuel, Morey and Sanes2018).
Selective deletion of Syt9 from rods showed effects only at higher flash intensities where cones contribute more significantly. However, deleting Syt9 from cones had no effect. Furthermore, deleting Syt9 from rods reduced photopic b-waves even though photopic responses are driven entirely by cones (Ronning et al., Reference Ronning, Allina, Miller, Zawadzki, Pugh, Herrmann and Burns2018) and eliminating Syt1 from cones is sufficient to abolish photopic ERG responses (Grassmeyer et al., Reference Grassmeyer, Cahill, Hays, Barta, Quadros, Gurumurthy and Thoreson2019). Rods and cones are coupled to one another via Cx36 gap junctions allowing rod signals to enter cone terminals where they can be transmitted to post-synaptic neurons (Fain & Sampath, Reference Fain and Sampath2018). Passage of signals the other way has not been carefully investigated but one possible explanation for these effects is that loss of Syt9 in rods somehow restricts the ability of rods to convey cone signals to downstream neurons. Since release from rods themselves is unimpaired, loss of Syt9 may somehow interfere with gap junction function, perhaps by altering protein trafficking as seen with other Syt isoforms (Wolfes & Dean, Reference Wolfes and Dean2020). Although we saw no gross anatomical changes, another possible explanation is that the loss of Syt9 from rods or other neurons may alter synaptic connectivity in subtle ways.
GABA and glycine are the major inhibitory neurotransmitter in the vertebrate retina (Eggers & Lukasiewicz, Reference Eggers and Lukasiewicz2011) and Syt9 has been shown to modulate GABA release in cultured striatal neurons (Xu et al., Reference Xu, Mashimo and Sudhof2007). These results led us to consider the possibility that loss of Syt9 might also interfere with GABA and/or glycine release from retinal neurons. Like effects of global deletion of Syt9, blocking GABAA receptors with bicuculline typically enhances ERG b-waves (Gottlob et al., Reference Gottlob, Wundsch and Tuppy1988; Arnarsson & Eysteinsson, Reference Arnarsson and Eysteinsson1997; Vitanova et al., Reference Vitanova, Kupenova, Haverkamp, Popova, Mitova and Wassle2001; Hirasawa et al., Reference Hirasawa, Miwa and Watanabe2021). By contrast, loss of GABAC receptors and application of GABAC antagonists have been found to reduce ERG b-waves (Kapousta-Bruneau, Reference Kapousta-Bruneau2000; Moller & Eysteinsson, Reference Moller and Eysteinsson2003; Wang et al., Reference Wang, Mojumder, Yan, Xie, Standaert, Qian, Pepperberg and Frishman2015). This suggests that if Syt9 is modulating GABA release, it is likely to be acting predominantly at synapses possessing GABAA receptors. Blocking glycine receptors with strychnine can also increase ERG b-waves (Arnarsson & Eysteinsson, Reference Arnarsson and Eysteinsson1997; Popova, Reference Popova2000), although this has not been seen in every preparation (Hirasawa et al., Reference Hirasawa, Miwa and Watanabe2021).
The OPs that ride on top of the ERG b-wave appear to arise from reciprocal synapses between rod bipolar cells and AII and A17 amacrine cells in the inner retina (Wachtmeister, Reference Wachtmeister1998; Liao et al., Reference Liao, Liu, Milla-Navarro, Villa and Germain2023). Loss of Syt9 from rods led to a reduction in scotopic OPs that paralleled reductions in scotopic b-waves. This suggests that diminished rod input accompanying loss of Syt9 led in turn to diminished amacrine cell activity. Global deletion of Syt9 had smaller effects on OPs, with a small increase in scotopic OPs at the highest intensities. The reduction in OPs produced by eliminating Syt9 from rods might be countered by increased amacrine cell activity mediated by actions of Syt9 in other cell types. Consistent with a contribution to shaping OPs from diminished GABA release in Syt9 global knockout mice, reducing GABAergic inhibition in GABAC receptor knockouts can also enhance OPs (McCall et al., Reference McCall, Lukasiewicz, Gregg and Peachey2002). Further studies on the sites and roles of Syt9 in the retina are needed to distinguish these various possibilities.
Funding
This study is funded by the NIH grants EY10542 and EY32396 to W.T. and T32NS105594 to C.S.M.
Competing interest
The authors declare no competing financial interests.