CVD is among the world's leading causes of death, with nearly 80 % of deaths occurring in developing countries(Reference Okrainec, Banerjee and Eisenberg1). Although cardiovascular death rates have significantly declined in most developed countries in the past decades, rates have grown in developing countries(Reference Yusuf, Hawken and Ounpuu2), such that 25–45 % of total deaths in these countries can be explained by CVD(Reference Yusuf, Reddy and Ounpuu3). Furthermore, it seems that people in developing countries experience coronary artery disease at a younger age than those in Western countries(Reference Reddy and Yusuf4). Therefore, identification of the determinants of cardiovascular risk factors is of particular importance in these countries.
Dietary fat intake has long been the source of interest in cardiovascular health. Among the different types of dietary fats, trans-fatty acids (TFA) are of high interest(Reference Mozaffarian, Katan and Ascherio5). Besides their natural dietary sources(Reference Chardigny, Malpuech-Brugere and Dionisi6), these fatty acids are formed during the partial hydrogenation of vegetable oils. Relative to unsaturated fatty acids, the intake of TFA results in higher LDL-cholesterol (LDL-C) concentrations, and relative to saturated fat, the intake of TFA results in lower HDL-cholesterol (HDL-C) concentrations(Reference Lichtenstein, Ausman and Jalbert7, Reference Judd, Baer and Clevidence8). A higher consumption of TFA has also been associated with an increased risk of developing CVD(Reference Oomen, Ocke and Feskens9) and type 2 diabetes(Reference Pisabarro, Sanguinetti and Stoll10). However, most information available in this regard has mainly been derived from studies done in Western countries, and it is unclear to what extent these findings apply to developing countries, as some data suggest that risk factors for CVD vary greatly between populations(Reference Yusuf, Hawken and Ounpuu2). Furthermore, the detrimental effects of trans-fats on lipid profiles, blood pressure and plasma glucose levels have been documented by short-term clinical trials with high doses of trans-fats, but limited observational studies have considered habitual consumption of partially hydrogenated vegetable oils (PHVO) and these outcome variables. This is particularly relevant for hypertension, where we have not found any observational study assessing PHVO in relation to blood pressure.
The average per-person home use of PHVO among Iranians is 14 g/4184 kJ (1000 kcal)(Reference Mozaffarian, Abdollahi and Campos11), with almost 33 % of fatty acids in these products as TFA(Reference Asgary, Nazari and Sarrafzadegan12). Therefore, Iranians take 4·2 % of their energy from TFA, almost twice the amount in developed countries(Reference Mozaffarian, Abdollahi and Campos11). Such dietary intakes might help to explain the high prevalence of cardiovascular risk factors, particularly high serum TAG levels and low serum HDL-C concentrations, among the Iranian population compared with that in Western populations(Reference Azizi, Esmaillzadeh and Mirmiran13). In the framework of dietary pattern analysis, we have shown that those with greater adherence to the Western and Iranian dietary patterns (both greatly loaded with PHVO) were more likely to have cardiovascular risk factors(Reference Esmaillzadeh and Azadbakht14). Furthermore, our previous investigations have demonstrated that consumption of hydrogenated vegetable oils (HVO) is independently associated with a greater risk of insulin resistance, the metabolic syndrome, elevated levels of markers of inflammation and endothelial dysfunction(Reference Esmaillzadeh and Azadbakht15, Reference Esmaillzadeh and Azadbakht16). In the present study, we aimed to evaluate the association of PHVO and non-HVO intake with individual cardiovascular risk factors.
Subjects and methods
Participants
Detailed information about the population and the measurements done can be found elsewhere(Reference Esmaillzadeh, Kimiagar and Mehrabi17–Reference Esmaillzadeh and Azadbakht19). Briefly, in the present cross-sectional study, performed among a representative sample of Tehrani female teachers aged 40–60 years, a total of 583 female teachers, selected by a multistage cluster random sampling method, were invited to participate and 521 women agreed (response rate: 89 %). To have a representative sample of female teachers, we divided twenty districts of Tehran Educational Offices into four categories (northern, western, southern and eastern parts of the city) because socio-economic variables are different in these four parts and dietary intakes of people living in these different parts might differ. Then, we randomly selected four districts (one from each category) of Tehran Educational Offices (districts 1, 9, 12 and 16). We obtained the list of schools (separately for public and private schools) and list of female teachers working in each district. Considering the number of public and private schools in each district, we randomly selected sixty-three schools (sixteen schools from district 1, seventeen schools from district 9, fourteen schools from district 12 and sixteen schools from district 16). The required number of female teachers was randomly selected proportionally to size in each district and school (131 women from district 1, 140 women from district 9, 113 women from district 12 and 137 women from district 16). After excluding women with a prior history of CVD, diabetes, cancer and stroke, those who had left>seventy items blank on the FFQ, those who reported a total daily energy intake outside the range of 3344–17 556 kJ (800–4200 kcal) and those taking medications that would affect serum lipoprotein, blood pressure and carbohydrate metabolism, 486 women remained for the present analysis. The whole project was approved by the Ethical Committee of the National Nutrition and Food Technology Research Institute, Shaheed Beheshti University of Medical Sciences. Informed written consent was obtained from each participant. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki.
Measurements
Usual dietary intakes were assessed by a trained dietitian using a validated semi-quantitative FFQ that consisted of a list of 168 food items with a standard serving size(Reference Esmaillzadeh and Azadbakht15). The frequency of consumption of a given serving of each food item during the previous year on a daily, weekly or monthly basis was asked. However, the reported frequencies were then converted to g/d using standard published guidelines(Reference Ghaffarpour, Houshiar-Rad and Kianfar20). PHVO (commonly used for cooking in Iran) was considered as the PHVO category. Sunflower oil, maize oil, rapeseed oil, soyabean oil and olive oil were defined as the non-HVO category. We did not collect data on the type of hydrogenated products that the participants consumed. Our FFQ included type of non-HVO individually, but only one item in our FFQ was about PHVO. Therefore, we were unable to describe the variety of PHVO consumed. However, the blending of oil from different sources, including sunflower, soyabean and other vegetable oils, to produce PHVO is a common practice in the developing world.
Weight, height and waist circumference were measured according to standard protocols, as described earlier(Reference Esmaillzadeh and Azadbakht19), and BMI was calculated. After a 12 h overnight fast, the blood sample was drawn for biochemical assessment. As described elsewhere in more detail(Reference Esmaillzadeh, Kimiagar and Mehrabi17, Reference Esmaillzadeh, Kimiagar and Mehrabi18), the analysis of samples was performed using a Selectra 2 auto-analyser (Vital Scientific, Spankeren, The Netherlands). Fasting plasma glucose was measured on the day of blood collection by an enzymatic colorimetric method using glucose oxidase (Pars Azmoon, Inc., Tehran, Iran). Serum TAG concentrations were assayed using TAG kits (Pars Azmoon, Inc.) by enzymatic colorimetric tests with glycerol phosphate oxidase(Reference Azadbakht, Atabak and Esmaillzadeh21). Serum HDL-C was measured after the precipitation of apoB-containing lipoproteins with phosphotungstic acid. Serum LDL-C was calculated from serum total cholesterol, TAG and HDL-C, except when the TAG concentration was greater than 4·4 mmol/l (4000 mg/l)(Reference Friedewald, Levy and Fredrickson22). The inter- and intra-assay CV of this method were < 10 %. Blood pressure was measured three times after the participants sat for 15 min, as reported earlier(Reference Esmaillzadeh, Kimiagar and Mehrabi17, Reference Esmaillzadeh, Kimiagar and Mehrabi18). Hypertriacylglycerolaemia was defined as serum TAG ≥ 2·2 mmol/l (2000 mg/l), hypercholesterolaemia as serum total cholesterol ≥ 6·24 mmol/l (2400 mg/l), high serum LDL-C as ≥ 4·1 mmol/l (1600 mg/l) and low serum HDL-C as < 1·29 mmol/l (500 mg/l)(23). Dyslipidaemia was defined based on the third report of the National Cholesterol Education Program Expert Panel(23) as having hypertriacylglycerolaemia, hypercholesterolaemia, high LDL-C or low HDL-C. Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg based on Joint National Committee VI(24). Diabetes mellitus was defined as fasting plasma glucose ≥ 6·93 mmol/l (1260 mg/l)(25). The presence of ‘at least one risk factor’ and ‘at least two risk factors’ of the three major risk factors for CVD (hypertension, dyslipidaemia and diabetes) was also evaluated. Data on physical activity, reported earlier in more detail(Reference Esmaillzadeh, Kimiagar and Mehrabi26), were obtained using the International Physical Activity Questionnaire(Reference Craig, Marshall and Sjostrom27) and expressed as metabolic equivalent-h/week. Additional covariate information regarding age, smoking habits, socio-economic status, medical history and current use of medications was obtained using questionnaires.
Statistical methods
First, we applied a residual method(Reference Willett28) to obtain energy-adjusted intakes of PHVO and non-HVO. Then, participants were categorised based on quintiles of energy-adjusted intakes of PHVO or non-HVO to reduce misclassification. ANOVA with Tukey's post hoc comparisons and χ2 tests were used to compare continuous and categorical variables, respectively, across quintiles. Dietary intakes of participants across quintiles of PHVO and non-HVO were assessed using ANCOVA, with age and energy as covariates. To obtain multivariate-adjusted means for cardiovascular risk factors across quintiles of PHVO and non-HVO, we controlled for the mutual effects of HVO and non-HVO (g/d), age (years), energy intakes (kJ/d), cigarette smoking (yes or no), physical activity (metabolic equivalent-h/week), socio-economic status (categorical), current oestrogen use (yes or no), menopausal status (yes or no) and family history of diabetes and stroke (yes or no) in the first model; further adjusted for dietary intakes (including intakes of cholesterol, fruits and vegetables, whole and refined grains, dairy, meat, fish and poultry all as continuous) in the second model; and additionally for BMI (continuous) in the final model to observe whether the associations are mediated by obesity. Multinomial logistic regression analyses (with the above-mentioned covariates) were used to calculate adjusted OR and 95 % CI by considering the sampling design. In all multivariate models, the first quintile was considered as the reference. The overall trend of OR was computed using the Mantel–Haenszel extension χ2 test. P < 0·05 was considered as significant. All statistical analyses were performed using SPSS (version 16.0; SPSS, Inc., Chicago, IL, USA).
Results
Mean energy-adjusted daily intakes of PHVO and non-HVO were 23 (sd 11) and 22 (sd 10) g/d, respectively. Characteristics of the study participants across quintile categories of PHVO and non-HVO are shown in Table 1. Those in the upper quintile of HVO had a higher age, BMI and waist-to-hip ratio and were more likely to be postmenopausal, while those in the upper quintile of non-HVO were younger and were more likely to have a family history of diabetes compared with those in the lowest quintile. There was no significant difference regarding the distribution of current smokers and obese people across quintile categories of either PHVO or non-HVO consumption. Those in the upper category of PHVO had higher intakes of cholesterol, while those in the top quintile of non-HVO had lower intakes of energy. Other nutrient intakes were not significantly different across quintile categories of either PHVO or non-HVO consumption. PHVO consumption was associated with higher intakes of high-fat dairy and lower intakes of low-fat dairy products, while non-HVO intake was associated with higher intakes of vegetables and low-fat dairy and lower intakes of high-fat dairy products.
Table 1 Characteristics of the study participants by quintiles of energy-adjusted amounts of partially hydrogenated (PHVO) and non-hydrogenated vegetable oils (HVO)*
(Mean values and standard deviations or percentages)
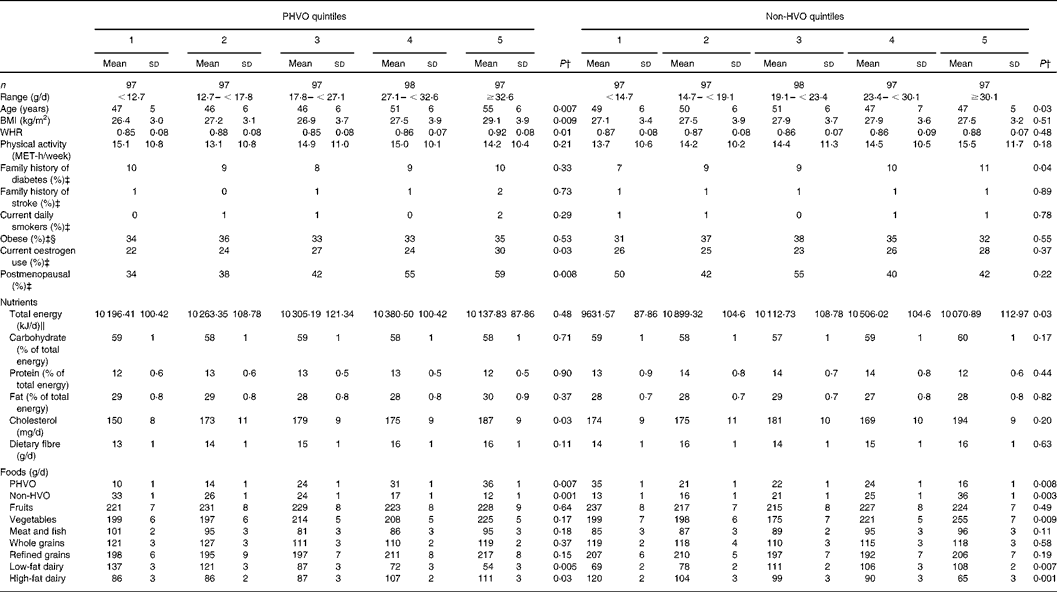
WHR, waist-to-hip ratio; MET, metabolic equivalent.
* Partially hydrogenated vegetable oil (PHVO, commonly used for cooking in Iran) was considered as the PHVO category. Sunflower oil, maize oil, rapeseed oil, soyabean oil and olive oil were defined as the non-HVO category.
† By using ANOVA for continuous variables and the χ2 test for categorical variables.
‡ Values are given in terms of percentages.
§ Obesity: BMI ≥ 30 kg/m2.
∥ 4·2 kJ (1 kcal) = 4·184 kJ.
Cardiovascular risk factors were more likely to be seen among individuals in the top quintile of PHVO intake compared with those in the lowest quintile (Table 2). In contrast, higher intakes of non-HVO were associated with a reduced prevalence of cardiovascular risk factors. The prevalence of diabetes across quintiles of PHVO and non-HVO consumption was not significantly different.
Table 2 Prevalence of cardiovascular risk factors across quintiles of partially hydrogenated vegetable oil (PHVO) and non-hydrogenated vegetable oil (HVO)*†
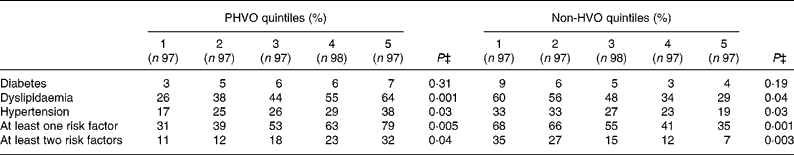
* Diabetes mellitus was defined as fasting plasma glucose ≥ 6·93 mmol/l ( ≥ 1260 mg/l). Dyslipidaemia was defined as having hypertriacylglycerolaemia ( ≥ 2·2 mmol/l or 2000 mg/l) or hypercholesterolaemia ( ≥ 6·24 mmol/l or 2400 mg/l) or high LDL-cholesterol ( ≥ 4·1 mmol/l or 1600 mg/l) or low HDL-cholesterol ( < 1·29 mmol/l or 500 mg/l). Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg. The presence of ‘at least one risk factor’ and ‘at least two risk factors’ was defined as having one or two risk factors from among the three major risk factors for CVD (hypertension, dyslipidaemia and diabetes), respectively.
† PHVO (commonly used for cooking in Iran) was considered as the PHVO category. Sunflower oil, maize oil, rapeseed oil, soyabean oil and olive oil were defined as the non-HVO category.
‡ χ2 test.
After controlling for age and other potential confounders, individuals in the top quintile of PHVO consumption had significantly higher concentrations of serum TAG, total- and LDL-C, elevated diastolic blood pressure and lower levels of serum HDL-C compared with those in the lowest quintile (data not shown). Further adjustment for dietary intakes including energy intake attenuated the associations. Even after further adjustment for BMI, the positive association of HVO consumption with serum TAG, total-, LDL- and HDL-C levels remained significant. Those in the highest quintile of non-HVO consumption had lower levels of serum TAG, total- and LDL-C, and higher levels of serum HDL-C compared with those in the lowest quintile. Further adjustment for dietary intakes removed the significant associations with serum HDL-C concentrations. However, even after additional adjustment for BMI, the inverse association with serum TAG and LDL-C levels remained significant (data not shown).
Multivariate-adjusted OR for individual cardiovascular risk factors across quintile categories of PHVO and non-HVO consumption are presented in Table 3. After controlling for age and other potential confounders, a high consumption of HVO was associated with a greater risk of having dyslipidaemia (OR for top v. bottom quintile 5·04; 95 % CI 2·70, 9·36), hypertension (OR for top v. bottom quintile 3·03; 95 % CI 1·55, 6·10), at least one (OR for top v. bottom quintile 8·52; 95 % CI 4·41, 16·41) and at least two risk factors (OR for top v. bottom quintile 3·60; 95 % CI 1·64, 7·74), while those in the top quintile of non-HVO consumption had lower odds for all these conditions. Further adjustment for dietary intakes had little impact on these associations. Even after additional adjustment for BMI, the positive association of HVO and the inverse association of non-HVO consumption with the above-mentioned cardiovascular risk factors remained significant, except for the association between non-HVO consumption and hypertension that became marginally significant. No overall significant associations were found between consumption of HVO and non-HVO and diabetes.
Table 3 Crude and multivariate-adjusted OR (95 % CI) for cardiovascular risk factors in Iranian women across quintiles of partially hydrogenated and non-hydrogenated vegetable oils*
(Odds ratios and 95 % confidence intervals)
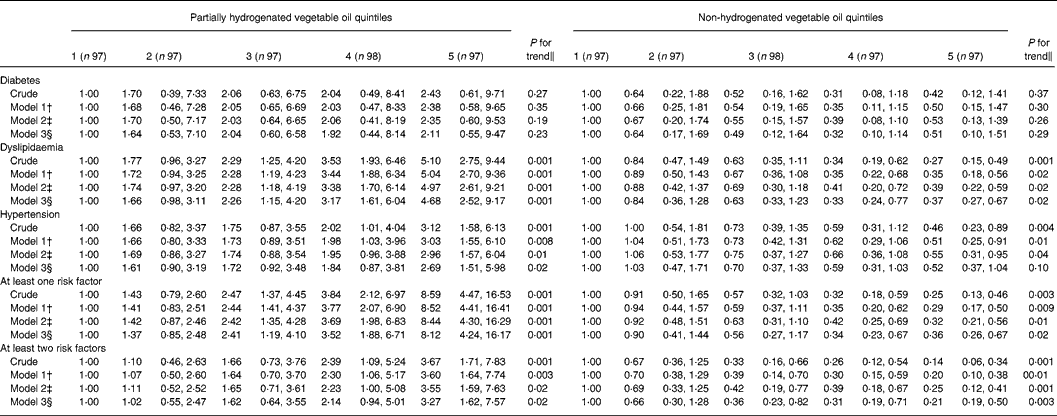
* Diabetes mellitus was defined as fasting plasma glucose ≥ 6·93 mmol/l ( ≥ 1260 mg/l). Dyslipidaemia was defined as having hypertriglyceridaemia ( ≥ 2·2 mmol/l or 2000 mg/l) or hypercholesterolaemia ( ≥ 6·24 mmol/l or 240 md/dl) or high LDL-cholesterol ( ≥ 4·1 mmol/l or 1600 mg/l) or low HDL-cholesterol ( < 1·29 mmol/l or 500 mg/l). Hypertension was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg. The presence of ‘at least one risk factor’ and ‘at least two risk factors’ was defined as having one or two risk factors from among the three major risk factors for CVD (hypertension, dyslipidaemia and diabetes), respectively.
† Model I: adjusted for the mutual effects of hydrogenated and non-hydrogenated vegetable oils (g/d), age (years), energy intakes (kJ/d), cigarette smoking (yes or no), physical activity (metabolic equivalent-h/week), socio-economic status (categorical), current oestrogen use (yes or no), menopausal status (yes or no) and family history of diabetes and stroke (yes or no).
‡ Model 2: further adjusted for dietary intakes including intakes of cholesterol, fruits and vegetables, whole and refined grains, dairy, meat, fish and poultry (all as continuous).
§ Model 3: additionally controlled for BMI (continuous).
∥ Mantel–Haenszel extension χ2 test.
Discussion
We observed a significant association between the consumption of PHVO and individual cardiovascular risk factors among a group of women in Iran. In contrast, non-HVO were inversely associated with cardiovascular risk factors. The mentioned associations persisted in the multivariate models accounting for known potential confounders.
The process of hydrogenation of vegetable oils, which prolongs the shelf life and alters the physical characteristics of vegetable oils, dates back to earlier findings about the detrimental effects of animal fat intake on human health(Reference McGandy, Hegsted and Myers29, Reference Keys, Anderson and Grande30). Subsequently, several investigators reported that hydrogenation results in greater amounts of TFA in HVO(Reference Lichtenstein, Ausman and Carrasco31). Higher intakes of TFA have been associated with an elevated risk of many chronic diseases(Reference Micha and Mozaffarian32). This is why the regulatory agencies in the USA and some European countries ruled the appearance of the TFA content of packaged foods on the Nutrition Facts panel(Reference Mozaffarian, Katan and Ascherio5). Although trans-fats occur in natural foods such as meat and dairy products, consumption of trans-fats in these products typically contributes less than 0·5 % of total energy intake(Reference Micha and Mozaffarian32). It seems that major dietary sources of trans-fats are industrially produced ones, particularly PHVO(Reference Azadbakht and Esmaillzadeh33). Until recently, PHVO were extensively used in Iran, probably due to governmental subsidy. However, due to adverse health effects of trans-fats on human health, the government has now changed its subsidy to non-HVO(Reference Azadbakht and Esmaillzadeh33).
Although earlier studies on Western populations have indicated the significant association of trans-fats and CHD risk(Reference Mozaffarian, Katan and Ascherio5, Reference Lichtenstein, Ausman and Jalbert7), limited data in this regard are available in developing countries. This is particularly important to consider as trans-fats contribute to a higher percentage of energy in developing countries as compared with developed nations(Reference Mozaffarian, Abdollahi and Campos11). In a case–control study in India, an elevated risk of IHD has been shown by vanaspati consumption which contains higher amounts of trans-fats(Reference Rastogi, Reddy and Vaz34). In Iran, based on the relationships of TFA intake with CHD incidence in prospective studies, it has been estimated that 19 % of CHD events would be prevented by half-reduction and 34 % would be prevented by near-elimination of TFA intake(Reference Mozaffarian, Abdollahi and Campos11). In Costa Rica, a positive significant association has been reported between elevated levels of TFA in adipose tissue and the risk of non-fatal acute myocardial infarction(Reference Baylin, Kabagambe and Ascherio35). However, the investigators failed to find a significant relationship between adipose tissue TFA levels and the increased risk of myocardial infarction after the industrial modification, which resulted in the reduction of TFA in the food supply of this population(Reference Colón-Ramos, Baylin and Campos36). The lack of an association between low levels of trans-fat intake and serum lipids has also been documented by others(Reference Aro, Kardinaal and Salminen37, Reference Roberts, Wood and Riemersma38). However, these findings, which are in contrast to ours, should not lead to a safe recommendation of trans-fat intake, because the findings of these studies were either based on individual trans-fats (rather than total trans-fats) or on the use of adipose tissue levels (rather than their intake). Totally, data on TFA consumption in developing countries are limited. However, it seems that PHVO are extensively used for cooking in these countries, making TFA contribution to total energy intake much greater than that in developed countries. Therefore, the findings of the present study are applicable to developing countries with a high intake of TFA. The present results might also be generalisable to other countries with moderate but still higher than the recommended intakes of TFA because previous studies have shown a dose-dependent relationship between TFA intake and the risk of CHD(Reference Mozaffarian, Katan and Ascherio5).
In the present study, a higher intake of PHVO was associated with a greater risk of having dyslipidaemia. Although the adverse effects of trans-fats on lipid profiles have extensively been studied in clinical trials, a few observational data have assessed the relationship between habitual consumption of trans-fats and serum lipid levels(Reference Mozaffarian, Aro and Willett39). To have a recommendation for the general population, observational data seem to be more important than clinical trials because most trials have evaluated the short-term effects of higher doses of trans-fat among healthy people, and the effects of habitual trans-fat intake have rarely been investigated. However, it must be kept in mind that associations found in observational studies, particularly those by cross-sectional studies, cannot ever be considered causal, and much evidence from different populations needs to be gathered before making public health recommendations. In a nested prospective study of American women, Sun et al. (Reference Sun, Ma and Campos40) found that higher erythrocyte trans-fat levels, as a biomarker of dietary intake of trans-fats, are associated with adverse lipid profiles. This finding is in accordance with the results of clinical trials in which isoenergetic replacement of either saturated or cis-unsaturated fats with TFA raised LDL-C and reduced HDL-C(Reference Micha and Mozaffarian32, Reference Mozaffarian, Aro and Willett39). The effects of a higher trans-fat intake on serum TAG and total cholesterol levels have also been documented(Reference Mozaffarian and Clarke41). Besides trans-fats, individuals with higher intakes of PHVO would have higher intakes of SFA and lower intakes of polyunsaturated fats. A greater SFA intake would result in an increase in plasma total cholesterol and LDL-C levels. Replacing these fatty acids with PUFA would decrease these lipid profiles(Reference Grundy and Denke42). Therefore, a high prevalence of dyslipidaemia among those with higher intakes of PHVO can also be explained by higher intakes of SFA and simultaneous lower intakes of PUFA. However, due to the limitation in the Iranian food composition table, we were unable to compute the exact dietary intakes of each individual fatty acid in this population.
We found that a higher intake of PHVO was associated with a greater risk of having hypertension, even after controlling for BMI. To the best of our knowledge, the present study is the first observational investigation reporting habitual PHVO intake in relation to blood pressure. Our observational finding is in contrast to what has been reported by clinical trials on the effects of trans-fats on blood pressure. A few trials, which have assessed such an impact, found no significant effects of trans-fat intake on blood pressure in healthy normotensive participants(43). This disagreement in findings can be explained by the short duration and small sample sizes in clinical trials. Other factors such as the cross-sectional design of the present study and residual confounding might account for this discrepancy. The mechanism by which TFA intake can affect blood pressure is unknown. However, it has been shown that consumption of TFA impaired endothelial function, as reflected by a reduction in brachial artery flow-mediated vasodilatation by 29 %(Reference Roos, Bots and Katan44). This dysfunction would in turn lead to elevated blood pressure.
We did not find any significant association between consumption of different kinds of vegetable oils, either PHVO or non-HVO, and the prevalence of diabetes, neither before nor after controlling for potential confounders. The finding of no significant association between PHVO and diabetes is in agreement with the studies of van Dam et al. (Reference van Dam, Rimm and Willett45) in the Health Professionals Follow-up Study and Meyer et al. (Reference Meyer, Kushi and Jacobs46) in Iowa. Both mentioned studies have not found significant association between trans-fat intake and incident diabetes. However, Hu et al. (Reference Hu, Manson and Stampfer47) found that trans-fat intake was significantly associated with diabetes incidence. The major findings from clinical trials also suggest that a higher trans-fat intake increases insulin resistance and diabetes risk(Reference Vega-Lopez, Ausman and Jalbert48). Our previous investigation among Iranian women also demonstrated that a higher PHVO intake is significantly associated with a greater risk of the metabolic syndrome and insulin resistance, while higher intakes of non-HVO were associated with a lower risk of insulin resistance(Reference Esmaillzadeh and Azadbakht16). The lack of a significant association between vegetable oils and diabetes in the present study needs further investigation. The possible reasons for this finding might be a lower prevalence of diabetes as compared with other cardiovascular risks, inadequate sample size and the cross-sectional design of the study.
Several limitations need to be considered in the interpretation of the present findings. The major limitation of the present study is its cross-sectional nature. Thus, the association between HVO consumption and cardiovascular risk factors in the Iranian population remains to be confirmed in prospective analyses. We have only considered the in-home consumption of HVO and non-HVO. This would probably underestimate the total intake and would affect the associations we studied in this population. As we used a FFQ for assessing dietary intakes, misclassification is a major concern in the present study as in any other epidemiological studies. The present study includes only women. It seems that Iranian women are at a greater risk of CVD as compared with men.
In conclusion, the present findings suggest that higher intakes of PHVO were associated with a greater risk of having cardiovascular risk factors, while higher intakes of non-HVO were associated with a lower risk of these conditions.
Acknowledgements
The data collection phase of the study was supported by a grant (P. 25/47/2337) from the National Nutrition and Food Technology Research Institute of the Islamic Republic of Iran and Shaheed Beheshti University of Medical Sciences, Tehran, Iran. The financial support for conception, design, data analysis and manuscript drafting comes from School of Public Health, Isfahan University of Medical Sciences, Isfahan, Iran. We thank the participants of the study for their enthusiastic support. None of the authors had any personal or financial conflicts of interest. A. E. and L. A. participated in the collection of data, conception and design, statistical analysis and data interpretation, manuscript drafting and approval of the final manuscript for submission.





