Oestrogen deficiency increases the risk of metabolic diseases, including type 2 diabetes, CVD, osteoarthritis (OA) and dementia, severely decreasing quality of life in many post-menopausal women. Abdominal fat accumulation is also involved in the aetiology and progression of metabolic diseases and acts both directly and indirectly by exacerbating the consequences of oestrogen deficiency(Reference Au, Feher and McPhee1). Oestrogen deficiency increases the release of inflammatory cytokines such as TNF-α, IL-1β and macrophage inflammatory protein-1(Reference Au, Feher and McPhee1) and indirectly exacerbates the inflammatory state by increasing abdominal fat(Reference Monteiro, Teixeira and Calhau2). Oestrogen protects against chronic inflammation. The prevalence of OA and Alzheimer’s disease increases in women by 2-fold after menopause(Reference Au, Feher and McPhee1). Women with bilateral oophorectomy at a young age, but not after menopause, are also at increased risk for dementia(Reference Phung, Waltoft and Laursen3,Reference Imtiaz, Tuppurainen and Tiihonen4) . As with Alzheimer’s disease, OA is directly associated with oestrogen deficiency and obesity and chronic inflammation induced by oestrogen deficiency is directly linked to its progression(Reference Ryuk, Ko and Lee5). Therefore, the elderly are recommended to decrease central fat stores and chronic inflammation to prevent and/or delay Alzheimer’s disease and OA(Reference Tamura, Omura and Toyoshima6).
Alzheimer’s disease and OA are important metabolic diseases that decrease the quality of life in elderly patients and their families. Due to the increase in life expectancy, many women will live with low oestrogen levels for the second half of their lives, putting them at increased risk of Alzheimer’s disease and OA(Reference Shin, Kang and Kim7). Interventions that result in an abdominal fat loss under well-controlled conditions may help protect against OA and Alzheimer’s disease with no adverse effects. Energetic restriction has positively impacted body fat, inflammation, cognitive function and OA(Reference Christensen, Henriksen and Leeds8–Reference Seimon, Roekenes and Zibellini11). However, the positive impacts are still controversial(Reference McNeill, Wu and Rabey12), and the consistent benefits of the energetic restriction method have been challenging to demonstrate, possibly due to poor compliance. Several intermittent fasting (IMF) regimes include complete alternative day fasting, modified fasting regimes, time-restricted feeding and religious fasting(Reference Patterson, Laughlin and LaCroix13). The IMF has recently shown better compliance and equivalent effects to continuous energy restriction for weight loss and decreasing waist and hip circumference, fat mass and dropout rates(Reference Seimon, Roekenes and Zibellini11). However, IMF reduces body fat without reducing daily energy intake, but it exacerbates hepatic insulin resistance in young rats(Reference Park, Yoo and Hyun14). IMF regimens have been shown to modulate energy, glucose and lipid metabolism, and inflammation to potentially influence OA and dementia(Reference Shin, Kang and Kim7,Reference Patterson, Laughlin and LaCroix13,Reference Liu, Cheng and Li15,Reference Yoon and Song16) . However, the IMF impacts on OA have not been studied, although a few Ramadan fasting-related studies have been conducted(Reference Ben Nessib, Maatallah and Ferjani17). It appears that Alzheimer’s-like disease (AD), OA, oestrogen deficiency and abdominal obesity, all have reciprocal actions, mostly mediated by systemic inflammation, that establish a vicious cycle that worsens all of the pathologies associated with OA and AD. Therefore, we decided to investigate whether a weight-loss approach could disrupt the cycle and used OA progression as the primary outcome to assess its success.
Ovariectomised (OVX) Sprague–Dawley rats aged over 8 weeks, when finishing puberty, have exhibited similar symptoms as menopausal women, but without unrelated symptoms of ageing(Reference Buniam, Chukijrungroat and Khamphaya18,Reference Leibowitz, Akabayashi and Alexander19) . When they have an amyloid-β injection in the hippocampus, they also develop Alzheimer’s disease-like symptoms(Reference Cui, Shan and Cao20); likewise, monoidoacetate (MIA) injection into their knee joints causes OA symptoms(Reference Yang, Kim and Qiu21). Therefore, OVX Sprague–Dawley female rats aged 10 weeks were chosen as an oestrogen-deficient animal model to examine the effects of diet and food restriction on AD exacerbated OA. IMF is shown to be effective for enhancing cognitive performance in elderly people with mild cognitive impairment(Reference Ooi, Meramat and Rajab22). A time-restricted feeding regime, early morning feeding type, was adopted to explore the IMF’s efficacy to suppress memory impairment and OA symptoms in the present study.
We hypothesised that Alzheimer’s-like disease would exacerbate OA and IMF with a high-protein (H-P) diet would reverse or halt memory impairment and OA symptoms in oestrogen-deficient animals with induced Alzheimer’s-like disease and OA. We examined the hypothesis in OVX rats with the hippocampal infusion of amyloid-β(25–35) and MIA injection into the articular cartilage of ad libitum or intermittently fasted rats with high fat (H-F) or H-P diets and explored the mechanisms involved.
Materials and methods
Animals and experimental design
Seventy-two Sprague–Dawley female rats aged 10 weeks (235 (sd 9) g) were purchased from DBL (Yeumsung-Kun, Korea). All rats were freely fed water and an American Institute of Nutrition-93G-based diet for a 1-week acclimation period. The rats were housed in individual stainless-steel cages in the conventional animal facility in a controlled environment (23 (sd 1) °C, 50 (sd 3) % humidity) under a 12-h light–12-h dark cycle (dark from 20.00 to 08.00 hours). All experimental procedures were approved by the Hoseo University Animal Care and Use Review Committee (HUACU-2014-07), according to the National Institute of Health guidelines for the care and use of laboratory animals.
Diets, including H-F and H-P, were made with a modified semi-purified American Institute of Nutrition-93 formulation(Reference Daily, Kang and Park23). The H-F consisted of 40 energy percentage (En%) carbohydrates, 17 En% protein and 43 En% fats, and the H-P contained 30 En% carbohydrates, 30 En% protein and 40 En% fats. All diets had similar energy densities, H-F (4·74 kcal/g) and H-P (4·64 kcal/g). The carbohydrate, protein and fat sources were starch plus sugar, casein plus methionine and lard plus soyabean oil (10:1; CJ Co.)(Reference Shin, Kang and Kim7). After the acclimation, experimental animals consumed water ad libitum and were provided their respective diets for the 6-week experimental period.
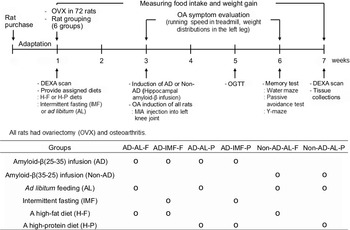
Seventy-two 11-week-old female Sprague–Dawley rats were randomly divided into six groups using rolling dice. Each group was blindly allocated into the assigned treatment groups before the surgery for OVX induction of menopausal symptoms. The experimental design is presented in Fig. 1. Each group included twelve OVX rats with MIA injection into the left knee articular cartilage, and they were housed in an individual cage. Each group was as follows: (1) hippocampal amyloid-β(25–35) infusion (AD) + ad libitum feeding (AL) with H-F (AD-AL-F), (2) AD + IMF with H-F (AD-IMF-F), (3) AD + AL with H-P (AD-AL-P), (4) AD + IMF with H-P (AD-IMF-P), (5) hippocampal amyloid-β(35–25) infusion (non-AD) + AL with H-F (non-AD-AL-F) and (6) non-AD + AL with H-P (non-AD-AL-P).
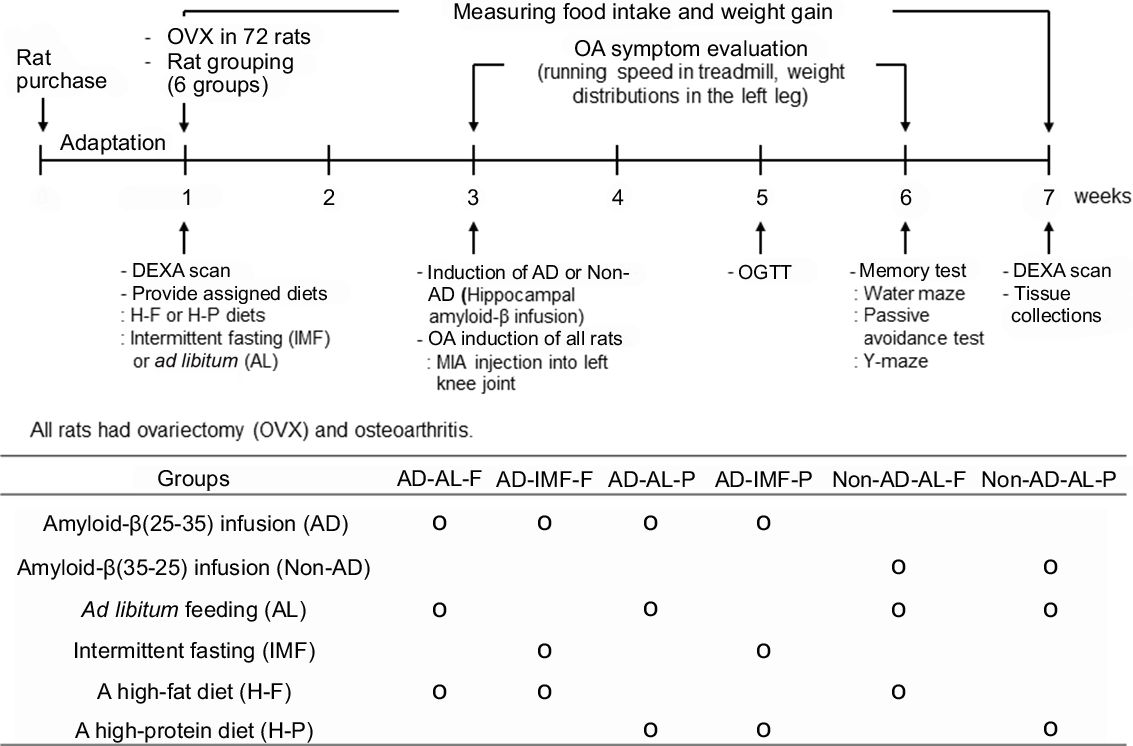
Fig. 1. Experimental design and grouping. All seventy-two rats had body composition measurements by dual-energy X-ray absorptiometry (DEXA), and they had ovariectomy (OVX). After all the rats had OVX, they were then randomly divided into six groups of twelve each. They consumed their assigned diets and followed their feeding methods for 2 weeks before AD and osteoarthritis induction, and then they had simultaneous AD and osteoarthritis inductions. They continued to receive their assigned diet feeding protocols for additional 4 weeks. Meanwhile, osteoarthritis symptoms were evaluated by observation of behaviours, running speed on a treadmill and weight distribution in the left leg every week. A glucose intolerance test was also assessed for glucose metabolism at the 6th week. Memory impairment was also determined by water maze, passive avoidance and Y maze at the 6th week. At the end of the experiment, all rats had DEXA scans for body composition determination. They were then randomly divided into six groups of twelve each: (1) AD-AL-F: hippocampal amyloid-β(25–35) infusion (AD) + ad libitum feeding (AL) + a high-fat diet (F), (2) AD-IMF-F: AD + intermittent fasting feeding (IMF) + F, (3) AD-AL-P: AD + AL + a high-protein diet (P), (4) AD-IMF-P: AD + IMF + P, (5) non-AD-AL-F: ICV amyloid-β(35–25) infusion (non-AD) + AL + F and (6) non-AD-AL-P: non-AD + AL + P.
All rats in each group had bilateral ovariectomies before the dark cycle began (14.00–18:00 hours). In brief, each ovary was ligated in the proximal part of each oviduct and removed with scissors after subcutaneously injecting the mixture of ketamine and xylazine (100 and 10 mg/kg body weight, respectively)(Reference Park, Lee and Seo24). They had either H-P or H-F and feeding types (IMF or ad libitum) for 2 weeks after OVX surgery. The four OVX groups (forty-eight rats) had hippocampal infusion with amyloid-β(25–35) as the AD group, and the two OVX groups (twenty-four rats) had ICV infusion with amyloid-β(35–25) (non-AD group). The amyloid-β(35–25) is a reverse sequence of amyloid-β(25–35) that does not form plaques in the brain (non-AD). Before the beginning of the dark cycle (14.00–18.00 hours), a stainless-steel cannula was implanted into the CA1 subregion of the anaesthetised rats in a stereotaxic device with the following coordinates: lateral, −3·3 mm from the bregma; posterior, 2·0 mm from the midline; ventral, –2·5 mm from the dura. The amyloid-β(25–35) and amyloid-β(35–25) were dissolved in sterile saline, and each solution was used to fill a separate osmotic pump (Alzet Osmotic Pump Company) at the rate of 3·6 nmol/d for 14 d. The stainless-steel cannula was connected to an osmotic pump filled with β-amyloid(25–35) for AD and (35–25) for non-AD. After inserting a cannula into the hippocampus, the anaesthetised rats had a single intra-articular injection of MIA (4 mg/50 μl saline; Sigma Co.) through the patellar ligament of the right knee, using a twenty-six-gauge needle(Reference Park, Park and Kim25). The diets were provided for an additional 4 weeks after ICV infusion of amyloid-β and MIA infusion into the joint.
In the IMF groups, rats had H-F or H-P for 3 h at the beginning of the dark cycle (19.00 to 22.00 hours), the mealtime of the rats corresponded to the morning for humans(Reference Park, Yoo and Hyun14). The 3-h feeding per d is similar to the 16-h fasting and 8-h feeding for humans in our previous and preliminary studies(Reference Park, Yoo and Hyun14,Reference Park, Zhang and Wu26) , since rats consume foods continuously, unlike humans. After MIA injections, clinical changes such as swelling, posture and behaviours were carefully observed and evaluated by a trained technician. Overnight-fasted serum glucose concentrations, food intake and body weight were measured every Tuesday at 10.00 hours. After 4 weeks of providing the assigned diets, an oral glucose tolerance test was conducted in overnight feed-deprived rats by oral administration of 2 g glucose/kg body weight. During oral glucose tolerance test, serum glucose concentrations were measured every 10 min until 90 min and at 120 min using a Glucometer (Accu-Chek; Roche Diagnostics) and serum insulin concentrations were measured at 0, 20, 40, 60, 90 and 120 min by an ELISA kit (Crystal Chem)(Reference Yang, Kim and Qiu21). Insulin resistance was assessed using the homoeostasis model assessment estimate of insulin resistance calculated by the previously provided equation(Reference Kim, Ko and Ryuk27). During the experiment, no rats died in any group. At the end of the study, rats injected insulin (5 U/kg body weight) into the inferior vena cava after anaesthetisation. Peri-uterine and retroperitoneal fat pads and uterine were dissected and weighed. Blood was collected by cardiac puncture, and serum was separated by centrifugation after allowing the blood to coagulate. Articular cartilage of the left joint (n 6) was dissected for measuring mRNA, and the rest of the left joint (n 6) was embedded with paraffin from each group. Serum and tissues were stored at –70°C for biochemical analysis.
Bone mineral density measurement
After calibrating a dual-energy X-ray absorptiometry (Norland pDEXA Sabre; Norland Medical Systems Inc.) with a phantom, bone mineral density (BMD), fat mass and lean body mass were measured in anaesthetised rats as previously described(Reference Kim, Ko and Ryuk27). Each rat was laid in a prone position, and the hip, knee and ankle articulations were at 90° flexion. After completing the scanning, BMD in the right femur and knee, and fat and lean mass in the abdomen, hip and leg were calculated using the appropriate dual-energy X-ray absorptiometry software.
Memory impairment measured by passive avoidance, Y maze and water maze tests
At the beginning of the dark cycle at 20.00 hours in the third week after amyloid-β infusion, the rats were assessed for short-term memory using a passive avoidance apparatus, a two-compartment dark/light shuttle box, as previously described(Reference Yang, Hwang and Kwon28). The short-term memory was measured by the retention latency time to enter the dark chamber when electric foot shock was not delivered. The latency time was recorded for a maximum of 600 s. Shorter latency time indicated memory impairment, compared with significantly longer latencies.
The next day after the passive avoidance test, rats were also subjected to a Y maze test consisting of a horizontal Y-shaped maze with three arms of 50·5 cm in length, 20 cm in width and 20 cm in height. Each rat was placed in one arm and monitored for movement in the Y maze for 8 min. If a rat consecutively entered into all three components, that was considered the right alteration. The percentage of the spontaneous correct alternations was calculated by the number of the right alternation among the total number of arm entries.
The acquisition of spatial memory was evaluated with a Morris water maze test, as previously reported(Reference Yang, Hwang and Kwon28,Reference Park, Kim da and Kang29) , at 2 d at the beginning of the dark cycle after the Y maze test. The water maze test measured the latency time to go to zone 5, where the platform existed, and the length of time to stay in zone 5 to find the podium during the third trial. The shorter latency time and longer staying time indicate better long-term memory and long-term spatial memory associated with hippocampal-dependent learning.
Progression of osteoarthritis and pain-related behaviour tests
At 20.00 hours in 3, 7, 14 and 21 d after MIA injection, all rats were carefully inspected to assess knee joint swelling and gait disturbances in the cages where they were allowed to move freely. Joint swelling and leg limping were evaluated as none (0), mild (1), moderate (2) and severe (3) symptoms in comparison with the no MIA injected rat by the same trained inspector who was blinded to treatment details throughout the study period(Reference Yang, Kim and Qiu21,Reference Park, Lee and Seo24) .
Pain-related behaviours were also measured by an incapacitance test using a hind limb weight-bearing apparatus (Linton Incapacitance Tester) at 7, 14 and 21 d after MIA injection and by the maximum running speed on a treadmill. The incapacitance tester is a device used to assess the differences in hind paw weight distribution between the right (osteoarthritic) and left (control) limbs due to the pain(Reference Park, Lee and Seo24). Animals were acclimated to the device for 30 min before the test, and weight distribution between two legs was measured five times in each rat, and the average of the middle three values was calculated. The results were represented as % weight distribution of right hind paw = right weight/(left weight + right weight) × 100(Reference Yang, Kim and Qiu21).
The maximum running speed was measured as a diagnostic criterion for OA. Rats ran at the initial speed of 40 cm/s for 1 min on the treadmill and then increased to 50 cm/s for 1 min at the beginning of the dark cycle. Its speed was increased by 5 cm/s every 1 min until the rats slid into the back of the treadmill(Reference Park, Lee and Seo24). The maximum speed for running was defined as the fastest speed that the rats could maintain for 20 s.
Gene expression in articular cartilage
Following the manufacturer’s instructions, the total RNA of the articular cartilage in the left joint (n 6) was individually extracted from each cartilage with Trizol reagent (Life Technologies). The cDNA was synthesised from 1 μg total RNA extracted from each rat using a superscript III reverse transcriptase kit (Life Science Technology). Specific genes associated with inflammation and degradation of the articular cartilage were amplified by mixing the cDNA of each articular cartilage, primers for the specific genes and SYBR Green mix (Bio-Rad) in duplicate using a real-time PCR instrument (Bio-Rad), as previously described. The primers for TNF-α, IL-1β, IL6, matrix metalloproteinase-3 and matrix metalloproteinase-13 genes were used(Reference Yang, Ko and Kwon30). The gene expression was quantified using the comparative cycle of threshold method described previously(Reference Yang, Kim and Qiu21).
Histopathological analysis of the knee
The paraffin-embedded left joints (n 6) were histologically examined to assess chronic morphological changes in knee articular bones for narrowing, loss of joint region, cartilage erosion and osteophyte formation(Reference Park, Park and Kim25,Reference Bar-Yehuda, Rath-Wolfson and Del Valle31,Reference Guzman, Evans and Bove32) . The left knee was collected for histological analysis, fixed with phosphate-buffered formalin, decalcified in 10 % nitric acid for 72 h and embedded in paraffin. Five-micrometre sections were stained with haematoxylin–eosin and safranin-O fast green. The trained inspector assessed morphological changes, including knee articular bones, cartilage erosion, loss of joint region and osteophyte formation in the stained sections(Reference Yang, Kim and Qiu21). The following scoring system for quantifying histopathological changes: Cartilage damage was evaluated on a scale of 0–5 where 0, no damage; 1, minimally affecting the superficial zone only; 2, mild invasion into the upper-middle area; 3, moderate invasion into the middle area; 4, marked invasion into the deep area but not to the tidemark and 5, severe full-thickness degradations to the tidemark. The extents of tibia plateau involvement and proteoglycan loss were scored as 1 (minimal), 2 (mild), 3 (moderate) and 4 (severe).
Statistical analysis
The study’s sample size was calculated at α = 0·05, β = 0·20, and effect size = 0·3 using G-power software (version 3.1.9.2), and the total number of animals was seventy-two. Each group contained twelve animals. Statistical analysis was conducted with SAS software version 7 (SAS Institute), and all results were expressed as means values and standard deviations. The results of the variables between AD-AL-F, AD-AL-P, AD-IMF-F and AD-IMF-P were analysed by two-way ANOVA to examine the statistical differences between IMF and diet types. The results between AD-AL-F, AD-AL-P, non-AD-AL-F and non-AD-AL-P were analysed with two-way ANOVA to assess the significance between AD presence and diet types. Multiple comparisons among all groups were conducted using Tukey’s test at P < 0·05.
Results
Body weight and visceral fat
At the initial stage, the body weight of each group was similar (263 (sd 3) g). The body weight gain for the first 2 weeks was reduced by the IMF before AD and OA induction, regardless of diets, and it was not affected by AD (Table 1). It was associated with decreased food intake in the IMF groups. During the last 4 weeks, the body weight gain did not differ among all groups, even though the IMF had less food intake than AD and non-AD. Decreased weight gain appeared to be linked to pain in all groups. Food intake was higher in AD-AL than non-AD-AL during the last 4 weeks, but it did not alter the weight gain. Diet types did not modulate food intake during the entire experimental period (Table 1). Visceral fat mass, including retroperitoneal and peri-uterine fat, was not significantly affected by AD with H-F, but IMF lowered visceral fat mass in both diets compared with AL (Table 1). Visceral fat mass was lower in non-AD-AL-P than AD-AL-P. IMF and diet types did not affect serum 17β-estradiol concentrations or uterine weights. However, tail skin temperatures, an index of hot flushing, was modulated by diet type and IMF. A high-protein diet and IMF decreased tail skin temperature. IMF with H-P prevented hot flush during menopause (Table 1).
Table 1. Body weight, visceral fat mass, serum 17β-oestradiol concentrations and tail skin temperature
(Mean values and standard deviations, n 12 for each group)
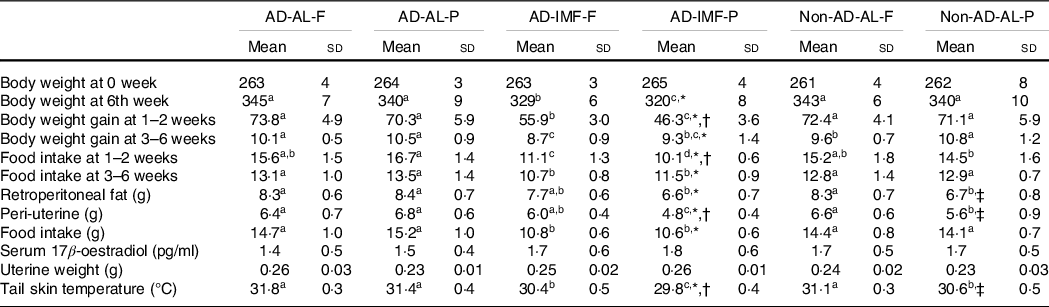
AD, hippocampal amyloid-β(25–35) infusion; AL, ad libitum feeding; F, high-fat diet; P, high-protein diet; IMF, intermittent fasting; non-AD, ICV amyloid-β(35–25) infusion.
* Significance with IMF by two-way ANOVA at P < 0·05.
† Significance with dietary protein in AD groups by two-way ANOVA at P < 0·05.
‡ Significance with AD by two-way ANOVA at P < 0·05.
a,b,cDifferent letters indicated significant differences among all the groups in the Tukey test at P < 0·05.
Inflammation, glucose and ketone metabolism
Serum TNF-α concentrations, an index of inflammation, were higher in the AD groups than in the non-AD group, and H-F increased them compared with H-P (Table 2). IMF decreased serum TNF-α concentrations compared with the AL. Serum cortisol concentrations also increased in the AD and AL compared with non-AD and IMF, respectively. H-F exacerbated the AD and AL effects on serum cortisol concentrations compared with the H-P (Table 2). IMF exhibited modestly but significantly higher serum concentrations of the ketone β-hydroxybutyrate, but the concentrations were not affected by AD and diet types.
Table 2. Metabolic parameters related to osteoarthritis symptoms
(Mean values and standard deviations, n 12 for each group)
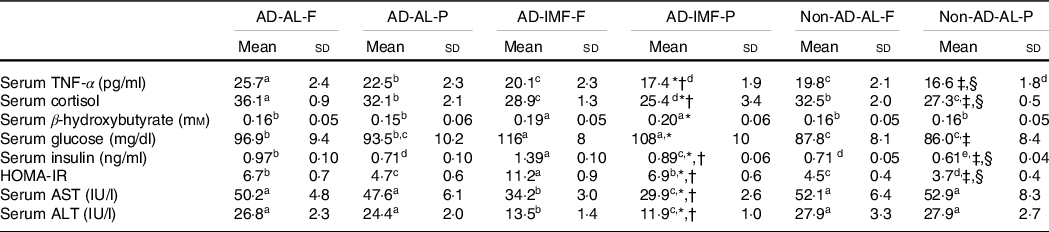
AD, hippocampal amyloid-β(25–35) infusion; AL, ad libitum feeding; F, high-fat diet; P, high-protein diet; IMF, intermittent fasting; non-AD, ICV amyloid-β(35–25) infusion; HOMA-IR, homoeostatic model assessment for insulin resistance; AST (GOT), aspartate aminotransferase; ALT (GPT), alanine aminotransferase.
* Significance with IMF by two-way ANOVA at P < 0·05.
† significance with dietary protein in AD groups by two-way ANOVA at P < 0·05.
‡ Significance with dietary protein in non-AD groups by two-way ANOVA at P < 0·05.
§ Significance with AD by two-way ANOVA at P < 0·05.
a,b,cDifferent letters indicated significant differences among all the groups in the Tukey test at P < 0·05.
Serum glucose concentrations at the fasting state increased in the order of non-AD-AL, AD-AL and AD-IMF, regardless of diet type (Table 2). Serum insulin concentrations also increased in non-AD-AL, AD-AL and AD-IMF, whereas the H-P diet decreased the concentrations compared with H-F. Homoeostasis model assessment estimate of insulin resistance, an index of insulin resistance, showed the same patterns (Table 2). IMF and AD increased homoeostasis model assessment estimate of insulin resistance compared with AL and non-AD, whereas H-P decreased it compared with a fat diet. Interestingly, serum GOT and GPT activities decreased in IMF groups compared with the AD groups, and the H-P diet lowered these activities (Table 2). Compared with AD-AL-P, AD-IMF-P reduced inflammation and liver damage, but it impaired glucose metabolism and insulin resistance.
Body composition
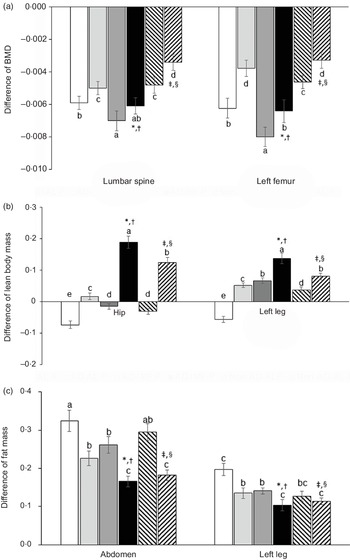
All animals had reduced BMD in the lumbar spine and femur since body composition, including BMD, was influenced by AD, IMF and diet types (Fig. 2(a)). Compared with non-AD, AD decreased BMD in both the lumbar spine and femur after the 6-week experimental periods, whereas IMF reduced BMD in both the lumbar spine and femur. H-P reduced the decrease of BMD by AD and IMF compared with H-F. The reduction of BMD was possibly linked to increased insulin resistance with AD and IMF (Fig. 2(a)).
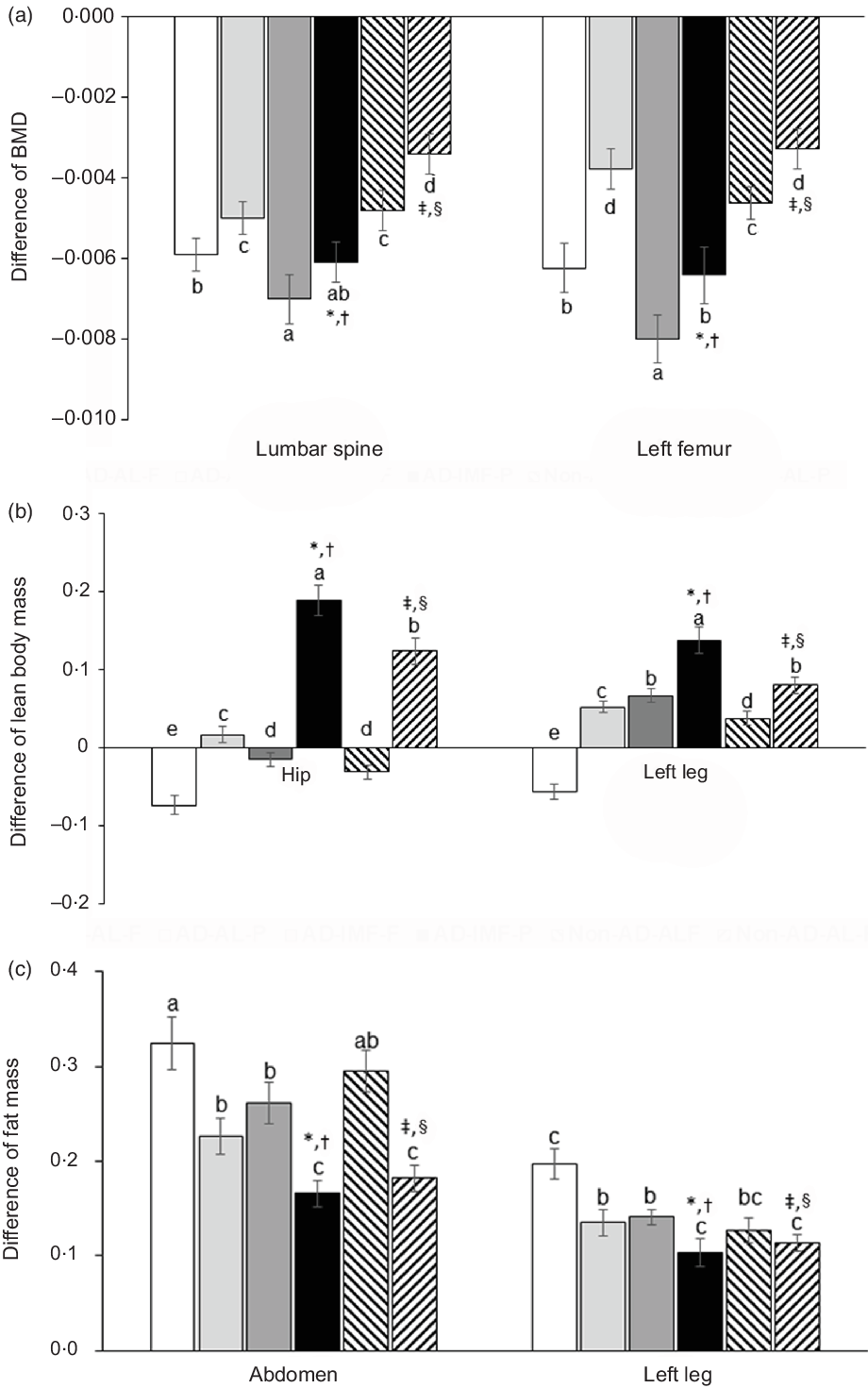
Fig. 2. Body composition of femur and knee with intra-articular injection of monoiodoacetate (MIA) at days 0 and 21 after an intra-articular injection of monoiodoacetate. (a) The differences in bone mineral density (BMD) in the lumbar spine, osteoarthritis (OA)-leg, and non-OA-leg before and after diet treatments. (b) The differences in lean body mass in the hip, OA-leg and non-OA-leg before and after diet treatments. (c) The differences in fat mass in the abdomen, OA-leg and non-OA-leg before and after diet treatments. Each data point and error bar represent the mean values and standard deviations from twelve rats per group. *Significant treatment effect among the groups by two-way ANOVA test by IMF at P < 0·05. †Significant treatment effect among the groups by two-way ANOVA test by dietary protein at P < 0·05. ‡Significant treatment effect among the groups by AD at P < 0·05. §Significance with dietary protein in non-AD groups by two-way ANOVA at P < 0.05. ![]() , AD-AL-F;
, AD-AL-F; ![]() , AD-AL-P;
, AD-AL-P; ![]() , AD-IMF-F;
, AD-IMF-F; ![]() , AD-IMF-P;
, AD-IMF-P; ![]() , non-AD-AL-F;
, non-AD-AL-F; ![]() , non-AD-AL-P. AD, hippocampal amyloid-β(25–35) infusion; AL, ad libitum feeding; F, high-fat diet; P, high-protein diet; IMF, intermittent fasting; non-AD, ICV amyloid-β(35–25) infusion.
, non-AD-AL-P. AD, hippocampal amyloid-β(25–35) infusion; AL, ad libitum feeding; F, high-fat diet; P, high-protein diet; IMF, intermittent fasting; non-AD, ICV amyloid-β(35–25) infusion.
AD reduced lean body mass in the hip and leg compared with the non-AD, but the increment with AD-IMF was more than non-AD groups, unlike BMD. H-P prevented the decrease of lean body mass in AD and IMF, compared with H-F (Fig. 2(b)). AD and IMF also lowered fat mass in the abdomen and leg, and its decrease in the abdomen and leg was similar to the non-AD (Fig. 2(c)). H-P decreased their fat mass more than H-F regardless of dietary patterns.
Memory impairment
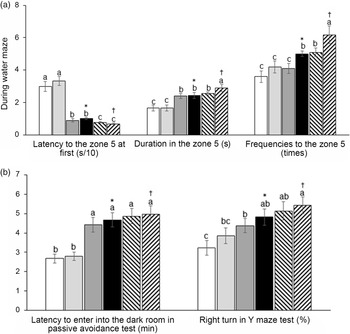
AD increased the latency to zone 5, where the platform was located at first compared with the non-AD regardless of diet type in the water maze test, and AD-IMF protected against the latency increase, making it close to the non-AD (Fig. 3(a)). The duration to stay in zone 5 to look for the platform was shorter in the AD group than the non-AD group, and AD-IMF made the duration in zone 5 longer, similar to the non-AD group. H-P increased the duration in zone 5 in non-AD compared with the non-AD-F (Fig. 3(a)). The frequencies to visit zone 5 showed a similar trend in the periods in zone 5. AD-IMF increased the frequencies to visit zone 5 compared with AL, and H-P elevated the frequencies compared with H-F (Fig. 3(a)). As a result, AD-AL decreased the memory function compared with non-AD-AL and AD-IMF protected against the memory impairment compared with AD-AL. Although diet types did not have a crucial influence on memory function, H-P improved memory function better than H-F. In the passive avoidance test, AD-AL exhibited a shortened latency to enter into the dark room at the third trial compared with non-AD-AL after experiencing the electric shock in the first two trials (Fig. 3(b)). IMF protected against the shortened latency in AD to as much as the non-AD, and diet type did not have influence the latency in the passive avoidance test (Fig. 3(b)). In the Y maze test, the percentage of right turns was lower in the AD-AL than non-AD-AL, whereas AD-IMF increased the percentage of consistent turns compared with the AD-AL. A high protein intake tended to increase the percentage compared with H-F, but it was not significantly different (Fig. 3(b)).
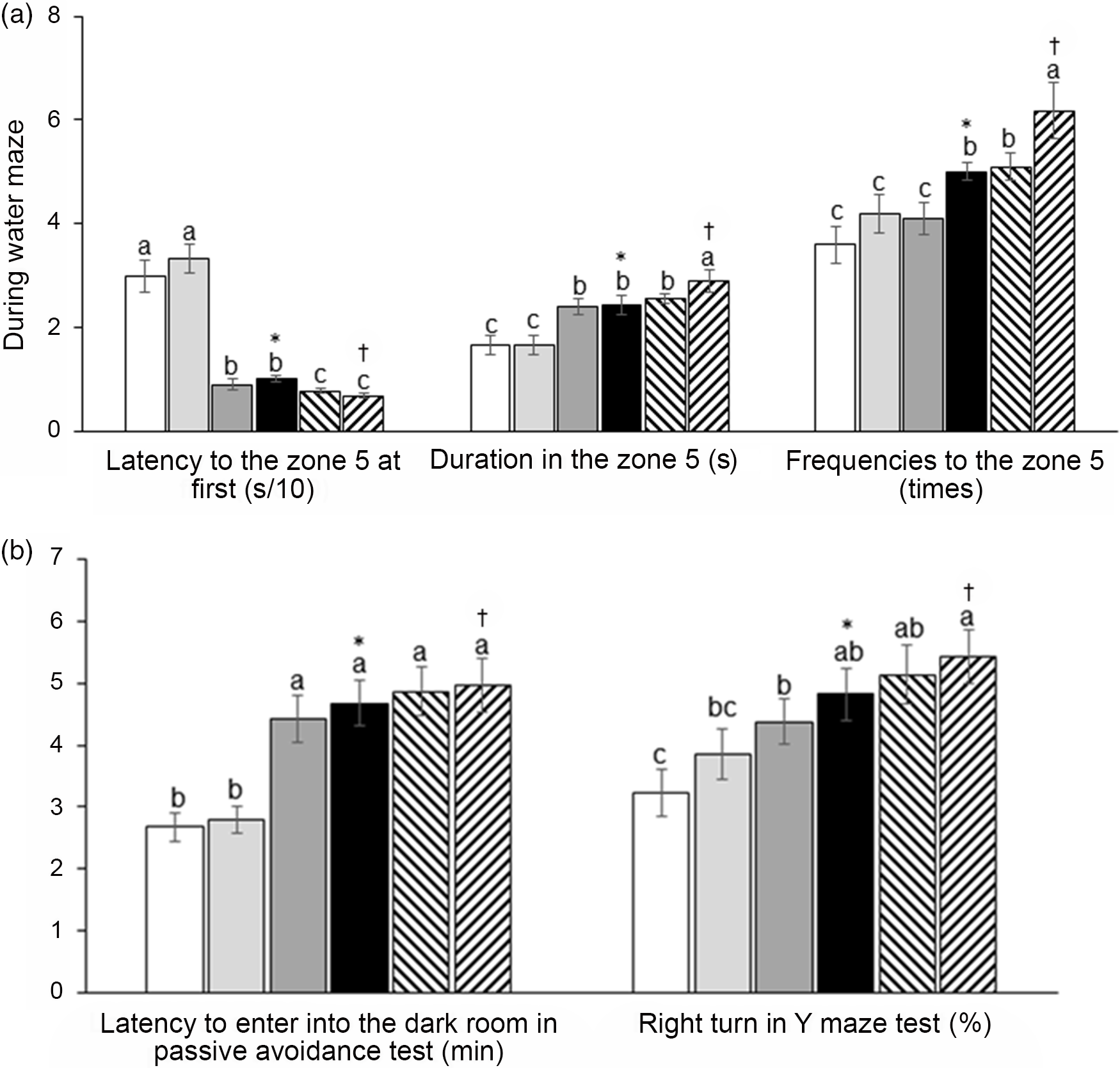
Fig. 3. Memory deficit of rats with an amyloid-β infusion. (a) The frequencies to visit the zone where the platform was located; duration to stay in the zone; the latency to visit the zone during a water maze test. (b) Latency time to enter the dark room in passive avoidance test and the number of the right turn in total turns in a Y maze test. Each data point and error bar represent the mean values and standard deviations from twelve rats per group. *Significant IMF effect among the AD groups by two-way ANOVA test at P < 0·05. †Significant AD effect among AL treatment groups by one-way ANOVA at P < 0·05. ![]() , AD-AL-F;
, AD-AL-F; ![]() , AD-AL-P;
, AD-AL-P; ![]() , AD-IMF-F;
, AD-IMF-F; ![]() , AD-IMF-P;
, AD-IMF-P; ![]() , non-AD-AL-F;
, non-AD-AL-F; ![]() , non-AD-AL-P. AD, hippocampal amyloid-β(25–35) infusion; AL, ad libitum feeding; F, high-fat diet; P, high-protein diet; IMF, intermittent fasting; non-AD, ICV amyloid-β(35–25) infusion.
, non-AD-AL-P. AD, hippocampal amyloid-β(25–35) infusion; AL, ad libitum feeding; F, high-fat diet; P, high-protein diet; IMF, intermittent fasting; non-AD, ICV amyloid-β(35–25) infusion.
Osteoarthritis symptoms
OA symptoms include swelling, limping, slower running speed and uneven weight distribution between the right and left legs. These symptoms are associated with inflammation and pain. The swelling of the knee is involved in inflammation, and it was not significantly different among the groups on day 2 (Fig. 4(a)). The swelling was reduced in all groups at different rates: AD increased the swelling more than non-AD, and the IMF prevented the swelling. IMF with H-P reduced the swelling the most, and AD-AL-F exhibited the highest swelling in the knee (Fig. 4(a)).
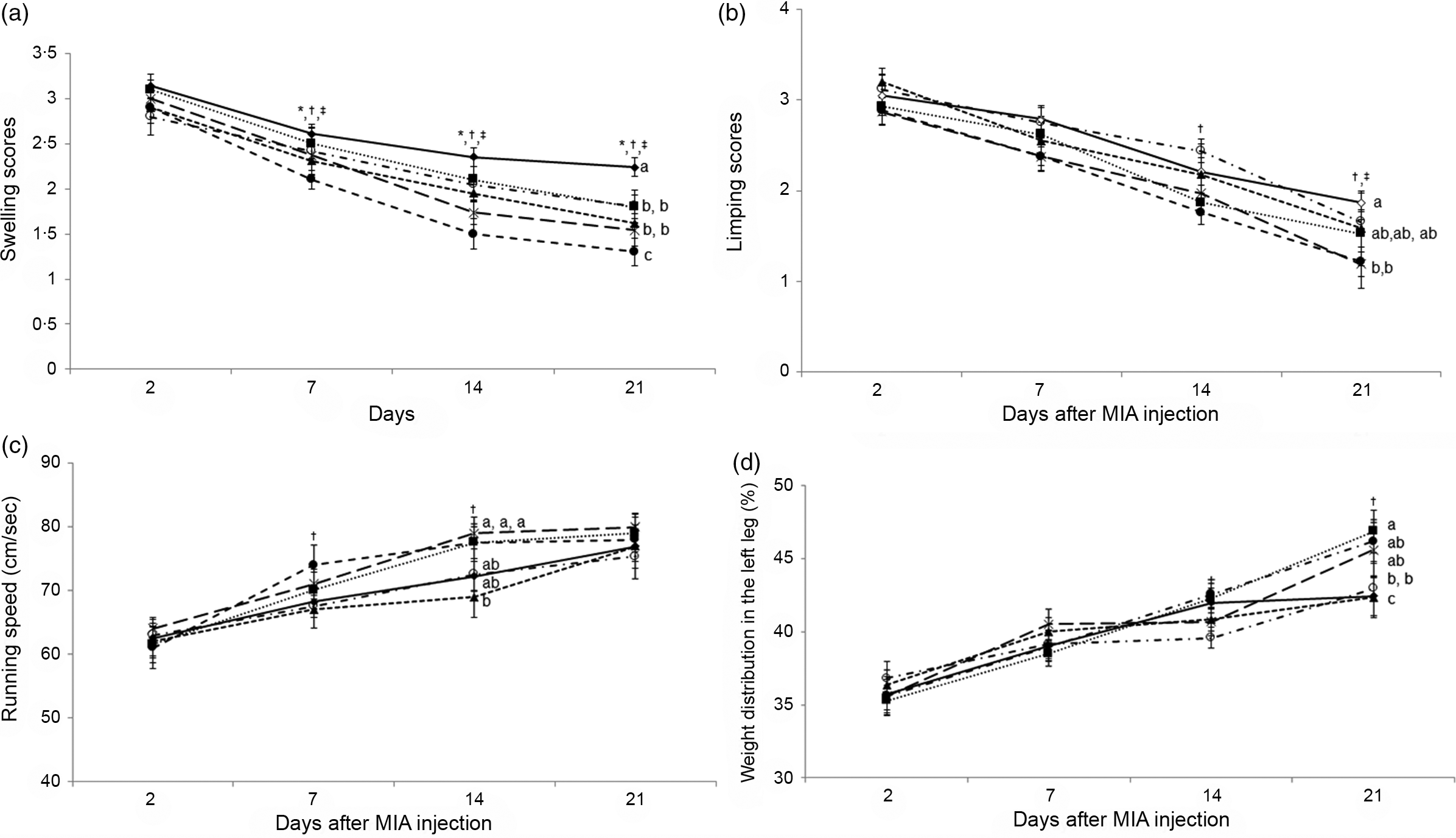
Fig. 4. Gross observation of osteoarthritis symptoms and pain-related behaviours at 3, 7, 14 and 21 d after an intra-articular injection of monoiodoacetate (MIA). (a) Oedema scores. (b) Limping scores. (c) The maximum velocity in treadmills. (d) Weight distribution between MIA- and saline-injected legs. Each data point and error bar represent the mean values and standard deviations from twelve rats per group. *Significant IMF effect among the AD groups by two-way ANOVA test at P < 0·05. †Significant dietary protein effect among the AD groups by two-way ANOVA test at P < 0·05. ‡Significant AD effect among AL treatment groups by one-way ANOVA at P < 0·05. ![]() , AD-AL-F;
, AD-AL-F; ![]() , AD-AL-P;
, AD-AL-P; ![]() , AD-IMF-F;
, AD-IMF-F; ![]() , AD-IMF-P;
, AD-IMF-P; ![]() , non-AD-AL-F;
, non-AD-AL-F; ![]() , non-AD-AL-P. AD, hippocampal amyloid-β(25–35) infusion; AL, ad libitum feeding; F, high-fat diet; P, high-protein diet; IMF, intermittent fasting; non-AD, ICV amyloid-β(35–25) infusion.
, non-AD-AL-P. AD, hippocampal amyloid-β(25–35) infusion; AL, ad libitum feeding; F, high-fat diet; P, high-protein diet; IMF, intermittent fasting; non-AD, ICV amyloid-β(35–25) infusion.
Pain scales are determined by assessing pain-related behaviours, including limping, running speed on a treadmill and weight distribution between legs. Limping of the legs was highest on day 2 and reduced over time in all groups (Fig. 4(b)). AD rats did not show significant differences in limping scores until day 14 after MIA injection into the joint, but AD increased limping at day 21 more than non-AD. IMF did not show differences in leg limping throughout the experimental periods. However, H-P reduced limping on days 14 and 21 after MIA injection into the joint (Fig. 4(b)). Interestingly, H-P markedly reduced the limping, and AD-IMF-P and non-AD-AL-P showed the lowest limping scores among the groups on days 14 and 21.
Running speed began to increase from day 2, and AD-IMF-P improved the running speed on day 7. A high protein intake resulted in the most significant increases in running speed at days 7 and 14 (Fig. 4(c)). Weight distribution showed similar results as running speed (Fig. 4(d)). H-P promoted equal weight distribution between both legs regardless of the AD and IMF diets. Therefore, the decrease of pain-related behaviours was associated with a high protein intake, but not AD and IMF. These results suggest that AD improvements might interfere with pain sensation to offset pain-related behaviours.
Joint histology and mRNA expression of articular cartilage
MIA injection into the left leg’s knee joint destroyed the articular cartilage and subchondral bone, and AD exacerbated the depth and extent of their damage as determined by the tide mark of the meniscus and joint space revealed by the haematoxylin–eosin staining (Fig. 5(a)). The larger scores of damage depth indicated that the tide mark was more deeply transmitted into the subchondral bone and that the damage had more extensively penetrated the meniscus. The depth and extent of damage were most extensive in the AD-AL-F among all groups. IMF and H-P prevented the damage to the tide mark of the meniscus and joint space (Fig. 5(a)). Articular collagen deterioration and articular damage, visualised by Safranin-O fast green staining, were exacerbated by AD-AL, but IMF partially prevented the decline. IMF and H-P caused a marked reduction of articular collagen deterioration and articular damage, compared with AL and H-F (Fig. 5(b)).
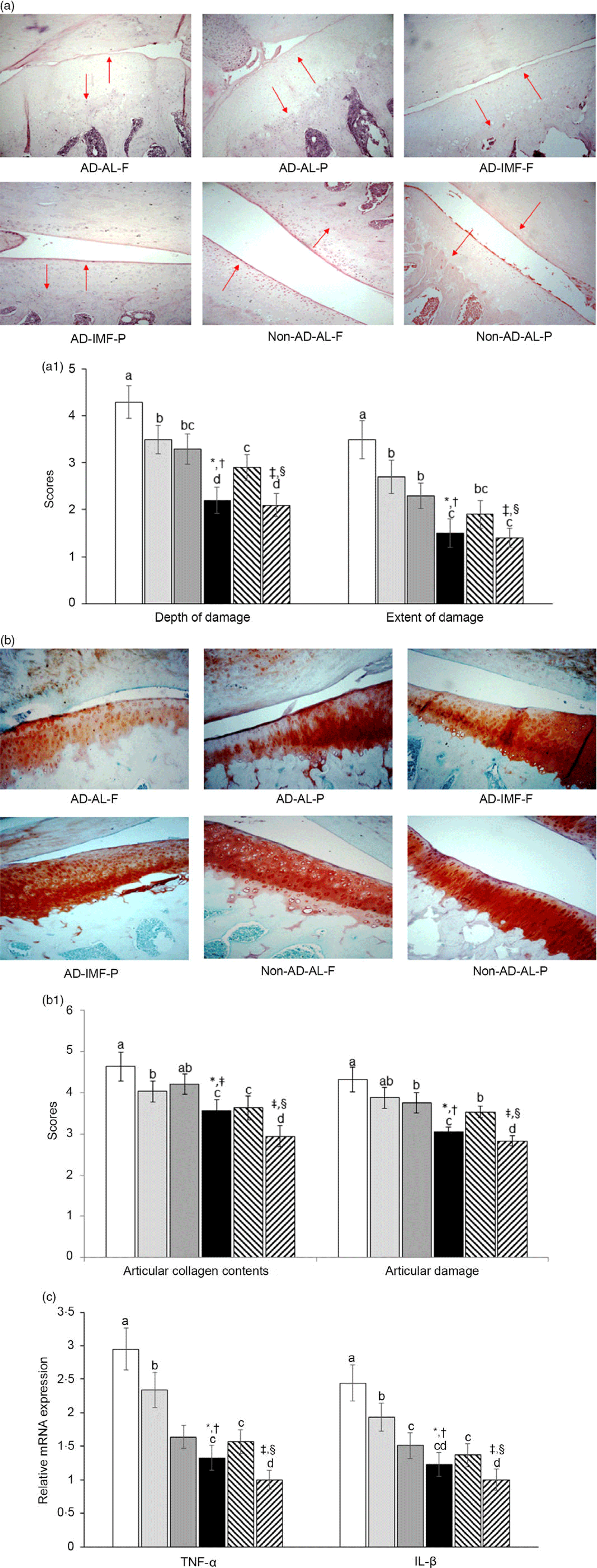
Fig. 5. The histopathological features of osteoarthritic lesions and the mRNA expression of matrix metalloproteinases (MMP) and proinflammatory cytokines in the articular cartilage at 28 d after an intra-articular injection of monoiodoacetate (MIA) (a) The damage to articular cartilage damage and subchondral bone in haematoxylin–eosin stain (magnifying power 100× and 200×). Red arrows indicated the damage extent of the articular cartilage. (b) The proteoglycan loss in the joint and extent of the tibial plateau in Safranin O–fast green stain (magnifying power 100×). (c) mRNA expression of MMP-3, MMP-13, TNF-α, IL-1β and IL-6 in the articular cartilage. Each data point and error bar represent the mean values and standard deviations from six rats per group. *Significant IMF effect among the AD groups by two-way ANOVA test at P < 0·05. †Significant dietary protein effect among the AD groups by two-way ANOVA test at P < 0·05. ‡Significant AD effect among AL treatment groups by one-way ANOVA at P < 0·05. § Significant dietary protein effect among AL treatment groups by one-way ANOVA at P < 0·05. ![]() , AD-AL-F;
, AD-AL-F; ![]() , AD-AL-P;
, AD-AL-P; ![]() , AD-IMF-F;
, AD-IMF-F; ![]() , AD-IMF-P;
, AD-IMF-P; ![]() , non-AD-AL-F;
, non-AD-AL-F; ![]() , non-AD-AL-P. AD, hippocampal amyloid-β(25–35) infusion; AL, ad libitum feeding; F, high-fat diet; P, high-protein diet; IMF, intermittent fasting; non-AD, ICV amyloid-β(35–25) infusion.
, non-AD-AL-P. AD, hippocampal amyloid-β(25–35) infusion; AL, ad libitum feeding; F, high-fat diet; P, high-protein diet; IMF, intermittent fasting; non-AD, ICV amyloid-β(35–25) infusion.
The mRNA expression of inflammatory cytokines, including TNF-α and IL-1β in the articular cartilage, was highest in the AD-AL-F among all groups (Fig. 5(c)). Like articular cartilage collagen scores, AD increased the mRNA expression, compared with non-AD, whereas it was reduced by the IMF and H-P, compared with AL and H-F (Fig. 5(c)).
Discussion
The prevalence of degenerative diseases in human populations is increasing due to longer life expectancy. The degenerative diseases, including Alzheimer’s diseases and OA, are interconnected, and their incidence increases in women 'after menopause(Reference Shin, Kang and Kim7). Energetic restriction improves cognitive function in overweight women and elderly subjects(Reference Witte, Fobker and Gellner33,Reference Dias, Santos and Magalhães34) , and IMF has been shown to attenuate neuroinflammation and memory dysfunction in animals systemically administered lipopolysaccharide(Reference Vasconcelos, Yshii and Viel35). In the present study, we explored the reciprocal interactions between AD and osteoarthritic symptoms and effects of IMF and diet composition on memory impairment and osteoarthritic joint disease progression in oestrogen-deficient animals fed H-F or H-P. The interconnection of memory and body composition was also explored. The study concluded that IMF with an H-P diet could alleviate memory impairment and OA symptoms in rats by increasing lean body mass and reducing the expression of proinflammatory cytokines in the articular cartilage. The results also indicated that neuroinflammation in AD rats exacerbated OA and vice versa.
Accumulating evidence indicates that chronic pain is associated with Alzheimer’s disease in the elderly population(Reference Aman, Pitcher and Ballard36). In a retrospective US cohort study, the elderly with OA were more likely to be diagnosed with Alzheimer’s disease and related dementia than those without OA, which was significant even after adjusting co-morbidities such as sociodemographics, lifestyles and medications(Reference Innes and Sambamoorthi37). Furthermore, chronic debilitating pain with and without OA is positively associated with memory impairment due to Alzheimer’s disease in the elderly after adjustment for multiple confounders(Reference Ikram, Innes and Sambamoorthi38). The positive relationship between OA and Alzheimer’s diseases is associated with pain and mood changes. It is also associated with a low-grade proinflammatory status in the elderly, called inflammaging(Reference Rezuș, Cardoneanu and Burlui39). Inflammaging is involved in degenerative diseases, including OA, sarcopenia and Alzheimer’s disease, and these diseases are interrelated to form a vicious cycle that exacerbates the symptoms of these diseases. Ramadan fasting reduces fat mass as well as serum concentrations of IL-6 and TNF-α, but not C-reactive protein in obese men(Reference Zouhal, Bagheri and Ashtary-Larky40) and animal models(Reference Shin, Kang and Kim7,Reference Park, Yoo and Hyun14) , indicating that decreasing adipose tissue stores may decrease systemic inflammation. The present study consistently demonstrated that IMF reduced TNF-α and IL-1β expressions in the articular cartilage in OA-induced rats. Therefore, the IMF was shown to alleviate the OA symptoms and ameliorate memory impairment(Reference Shin, Kang and Kim7,Reference Park, Zhang and Wu26) , and it may be related to the reduction of inflammation, as indicated by lower serum TNF-α concentrations.
Although the AD group had less pain sensation, inflammation in the joint injected with MIA was high. The expressions of proinflammatory cytokines in the articular cartilage were higher with AD and lowered with IMF and H-P. Their expressions were highest in the AD-AL-F group. Early time-restricted feeding (eating between 08.00 and 14.00 hours) in humans resulted in decreased serum glucose concentrations during 24-h glucose monitoring and increased serum ketone and cholesterol concentrations before starting food intake in a randomised clinical trial(Reference Jamshed, Beyl and Della Manna41). Early time-restricted feeding in men with prediabetes also improved insulin sensitivity, blood pressure and oxidative stress without body weight changes(Reference Sutton, Beyl and Early42). Obesity and associated metabolic syndrome pathologies are well-known risk factors for OA. The metabolic syndrome directly deteriorates articular cartilage by stimulating the generation of proinflammatory cytokines such as TNF-α and IL-4 and catabolic factors such as matrix metalloproteinase-3 and matrix metalloproteinase-13 in inducing and exacerbating OA(Reference Dickson, Roelofs and Rochford43,Reference Shen, Zhao and Shang44) . The metabolic syndrome indirectly exacerbates OA symptoms by increasing free fatty acids and advanced glycation end-products that activate macrophages, damaging the articular cartilage(Reference Dickson, Roelofs and Rochford43). AD increased insulin resistance in the present study, which exacerbates OA symptoms by disrupting the brain–liver axis(Reference Daily, Kang and Park23,Reference de la Monte, Longato and Tong45) . IMF is known to decrease fat mass, reduce the metabolic syndrome(Reference Faris, Jahrami and Alsibai46) and indirectly decrease OA symptoms. Therefore, the IMF can mitigate OA symptoms directly and indirectly.
Diet types also influence OA symptoms by changing muscle mass and strength(Reference Zhang, Pan and Deng47). In a cross-sectional study, the participants with knee OA had lower protein intakes than the daily recommended intake and lower protein intake was associated with lower muscle strength and muscle mass in the elderly(Reference de Zwart, van der Leeden and Roorda48,Reference Bae and Kim49) . The longitudinal Framingham Osteoarthritis Study demonstrated that the participants with higher dietary fibre intake (≥21 g/d) had fewer knee OA symptoms by 0·7 times than those with lower dietary fibre intakes(Reference Dai, Jafarzadeh and Niu50). This association was related to BMI but not C-reactive protein. The study suggests that fibre intake has the protective activity on knee OA which is partly mediated by BMI. In the present study, the IMF and H-P also mitigated OA symptoms associated with decreased body weight. On days 14 and 21, H-P improved running speed and weight distribution in both legs regardless of eating patterns and AD. Furthermore, OA development is inversely associated with the muscle mass related to protein supplementation and exercise in the elderly(Reference Liao, Wu and Tsauo51). Thus, dietary regimens can increase muscle mass and strength to mitigate OA.
The present study had the merit to examine the integration of several diseases in the elderly. However, each disease status was induced by surgical interventions and may not be entirely representative of the related disorders in elderly people, although each induced disease in rats showed similar symptoms as a human disease(Reference Cui, Shan and Cao20,Reference Yang, Kim and Qiu21,Reference Kim, Ko and Ryuk27) . The present study was designed to show the interaction of inflammation between the peripheral tissues and the brain, even though they are not directly connected.
In conclusion, AD exacerbated the articular cartilage deterioration despite no elevation in pain-related behaviours. AD exacerbated osteoarthritic symptoms and vice versa, and IMF with H-P alleviated memory impairment and OA symptoms by increasing lean body mass, decreasing fat mass and reducing proinflammatory cytokine expression. These results suggest that AD patients may need regular medical check-ups for OA development and progression. The results support recommendations that elderly persons with OA reduce body weight by dietary regimens such as IMF with proper protein intake to maintain muscle mass and strength. However, although human research suggests that IMF is safe and effective for the elderly(Reference Ooi, Meramat and Rajab22), it should be used cautiously and with medical supervision, especially in people with impaired blood glucose regulation. Further randomised clinical trials or prospective studies are needed to confirm the results of this study.
Acknowledgements
This study was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2019R1A2C1007203).
Conceptualisation, methodology, resources, supervision, writing-original-draft preparation: S. M. P.; formal analysis, data curation and writing-review and editing: B. K. S. All the authors have read and agreed to the published version of the manuscript.
The authors declare that they have no conflicts of interest.