A high plasma cholesterol level has been clearly identified as a risk factor for atherosclerosis and CVD(Reference Chobanian1). Additionally, high plasma concentrations of LDL cholesterol can favour the development of CVD(Reference Drake, Hannani, Fei, Lavi and Berliner2). Oxidative modification of LDL also plays an important role in the development of atherosclerosis(Reference Steinberg3) and this oxidized LDL has been found in atherosclerotic lesions(Reference Esterbauer, Gebicki, Puhl and Jurgens4).
Oxidized LDL possesses various biological activities, such as increasing the accumulation of lipids in macrophages(Reference Sparrow, Parthasarathy and Steinberg5), stimulating the chemotaxis of circulating monocytes(Reference Berliner, Territo and Sevanian6) and modulating the expression of various growth factors, adhesion molecules and cytokines(Reference Rajavahira, Andalibi, Territo, Berliner, Navab, Fogelman and Lusis7). Oxidative modification of LDL induces the formation of immunogenic epitopes in the LDL molecule, which leads to the formation of antibodies against oxidized LDL that can be detected in serum(Reference Palinski, Rosenfeld and Yla-Herttuala8). These levels of anti-oxidized-LDL antibodies have been proposed as a method of directly estimating the oxidized LDL antigen in vivo (Reference Palinski, Rosenfeld and Yla-Herttuala8, Reference Craig9), although others have found that the levels of these antibodies have no direct association with the amount of oxidized LDL(Reference Karabinos, Koulouris and Melpidou10). While many observational studies have shown direct associations between anti-oxidized-LDL antibodies and established atherosclerosis(Reference Lehtimaki, Lehtinen and Solakivi11, Reference Inoue, Uchida and Kamishirado12), others have not(Reference Shoji, Nishizawa and Fukumoto13).
The oxidation of LDL is a process of lipid peroxidation during which the phospholipid PUFA in the lipoproteins are attacked by free radicals(Reference Yla-Herttuala, Palinski and Rosenfeld14). Susceptibility of LDL to peroxidation may therefore partly depend on its compositional characteristics of the antioxidant and fatty acid content. An increase in linoleic acid leads to a rise in oxidation of the LDL ex vivo and macrophage degradation of LDL, as compared with a diet rich in oleic acid(Reference Abbey, Belling, Noakes, Hirata and Nestel15, Reference Reaven, Parthasarathy, Grasse, Miller, Steinberg and Witztum16). Other studies have looked at the association between the different fatty acids in the diet and the susceptibility of the LDL to oxidation. However, the relation between levels of antibodies against oxidized LDL and the fatty acid composition of the serum phospholipids has received little attention. Gradek and co-workers noted decreased levels of anti-oxidized-LDL antibodies during the postprandial period in patients with atherosclerosis when the medium was enriched with PUFA(Reference Gradek, Harris, Yahia, Davis, Le and Brown17). Nevertheless, another study found that fatty meals did not reduce the antibodies in healthy patients(Reference Le, Li, Kyung and Brown18).
The aim of this study was to examine the association between the levels of antibodies and oxidized LDL and the various fatty acids in the serum phospholipids in women.
Materials and methods
Subjects
The study was undertaken in Pizarra, an urban town in the province of Malaga, Andalusia, southern Spain, founded in 1818 and composed of ethnically homogeneous persons of Caucasian origin.
A total of 465 women aged 18–65 years, selected randomly from the municipal census, were included in the study. All institutionalized persons, for whatever reason, were excluded from the study, as were subjects with known diabetes mellitus, pregnant women, and those persons with a severe clinical problem or psychological disorder; basically, persons were excluded if they were unable to attend without assistance the clinic where the study was undertaken or if they did not have the legal capacity to sign the informed consent. The subjects were requested by mail to attend their local health centre for a medical examination. Those who failed to attend their first appointment were sent a second letter giving them another appointment, and all those still not attending were visited at home in order to ascertain the reason. Thus, the final study population included 391 women.
All subjects were informed of the nature of the study and gave their written consent to participate. The study was approved by the Ethics and Clinical Research Committee of Carlos Haya Hospital.
Procedures
All participants were interviewed and given a standardized clinical examination by the same researchers. The clinical data included weight, height, BMI (kg/m2) and waist:hip ratio. Venous blood samples were taken after a minimum of 10 h fasting. The serum was separated immediately after extraction and stored at − 80°C until analysis. Measurements were also made of glucose, cholesterol, TAG and HDL-cholesterol by enzymatic methods using a Dimension autoanalyzer (Dade Behring Inc., Deerfield, IL, USA). LDL-cholesterol was calculated from the Friedwald equation.
Fatty acid composition of serum phospholipids
For fatty acid analysis, lipids were extracted with chloroform–methanol (2:1, v/v)(Reference Folch, Lees and Sloane Stanley19). The lipid classes were separated by TLC with hexane–ethyl ether–acetic acid (80:20:2, by vol.) as the developing solvent. Fatty acid methyl esters of phospholipids were prepared according to Lepage & Roy(Reference Lepage and Roy20) and analyzed in a Hewlett–Packard 4890A gas chromatograph equipped with a Supelco OMEGAWAX™ 320 flame ionization detector and capillary column (30 m × 0·32 mm × 0·25 μm film thickness). The oven temperature was maintained at 140°C for the first minute and increased at a rate of 6°C per min until 240°C. This temperature was maintained for 4 min.
Oxidized LDL
LDL was isolated from a pool of fasting plasma from human blood donors by density gradient ultracentrifugation at 65 000 rpm (BECKMAN Optima XL100K ultracentrifuge, vertical rotor NVT65.2) for 35 min at 4°C. This was then further purified with a second ultracentrifugation at 49 000 rpm (fixed angle rotor 70.1) for 18 h at 4°C. The LDL was then dialyzed against PBS (4°C for 30 h; 0·14 m-NaCl–0·01 m-phosphate buffer), obtaining the native LDL. Oxidized LDL was prepared by incubating the native LDL for 3 h at 37°C with 0·5 m-malonyldialdehyde (MDA) at a constant ratio of 100 μl per mg LDL. The reaction was stopped by adjusting the pH to 7·4 with 1 m-NaOH. After conjugation, oxidized LDL (MDA-LDL) was extensively dialyzed against PBS.
Anti-oxidised-LDL antibodies
Microtitre plates for determination of anti-MDA-LDL antibodies were coated with either native LDL or MDA-LDL, both at 10 mg/ml in PBS. The plates were incubated for 2 h at 37°C and overnight at 4°C. After washing four times with PBS, the plates were blocked with 1 % bovine serum albumin/PBS for 2 h at room temperature. Serum samples were diluted 1:100 in 1 % BSA/PBS and incubated for 3 h at room temperature. After washing, an alkaline phosphatase-conjugated antihuman IgG (Sigma Immuno Chemical, St Louis, MO) was diluted 1:1000 in 1 % bovine serum albumin/PBS and added. It was then left for 3 h at room temperature. One mg/ml p-nitrophenyl-phosphate (Sigma) in 500 mm-carbonate buffer containing 1 mm-MgCl2 (pH 9·8) was used as substrate. The reaction was stopped after 60 min with 1 m-NaOH. The absorbance was read in an ELISA reader (Labsystem Multiskan, MS, Helsinki, Finland).
Duplicate determinations were performed for each serum sample. The binding of antibodies to MDA-LDL (anti-oxidized-LDL antibodies) was calculated by subtracting the binding of native LDL from the binding of MDA-LDL. The results were expressed as an optical density. The inter- and intra-assay CV of the technique ranged from 5 to 15 %(Reference Tinahones, Gomez-Zumaquero and Rojo-Martinez21).
Statistical analysis
The data are presented as the means with standard deviation for continuous variables and as proportions for discrete variables. Spearman's correlation coefficients were calculated to test for associations between different variables. Multiple regression analysis was used to study which variables were associated with the variability of the anti-oxidized-LDL antibodies. In all cases, the rejection level for a null hypothesis was α = 0·05 for two tails. Analyses were made using SPSS version 10 (SPSS Inc., Chicago, IL, USA).
Results
Table 1 shows the main anthropometric and laboratory values in the overall sample. The levels of anti-oxidized-LDL antibodies correlated significantly with age (r − 0·341, P < 0·001), BMI (r − 0·239, P < 0·001), waist:hip ratio (r − 0·285, P < 0·001), glucose (r − 0·208, P < 0·001), cholesterol (r − 0·243, P < 0·001), LDL-cholesterol (r − 0·185, P = 0·002), serum phospholipid EPA (r − 0·159, P = 0·003), DHA (r − 0·121, P = 0·026), and the sum of the n-3 PUFA (r − 0·141, P = 0·009). The levels of anti-oxidized-LDL antibodies did not correlate significantly with the levels of TAG, HDL-cholesterol, the sum of the SFA, serum phospholipid myristic acid, serum phospholipid palmitic acid, serum phospholipid linoleic acid, serum phospholipid arachidonic acid or the sum of the serum phospholipid n-6 PUFA.
Table 1 Biological variables in the study group (n 391)
(Means and standard deviations)
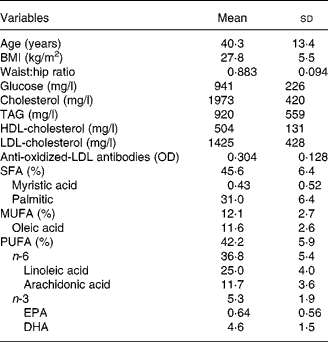
OD, optical density.
The multiple regression analysis showed that the variables explaining the behaviour of the levels of anti-oxidized-LDL antibodies were age (P < 0·001) and the serum phospholipid EPA levels (P < 0·001). Other variables that were included in the model but were not associated with the levels of anti-oxidized-LDL antibodies were BMI (P = 0·641), waist:hip ratio (P = 0·305), glucose (P = 0·433), cholesterol (P = 0·250), LDL-cholesterol (P = 0·649), HDL-cholesterol (P = 0·119), myristic acid (P = 0·606), arachidonic acid (P = 0·513), oleic acid (P = 0·779), linoleic acid (P = 0·337), palmitic acid (P = 0·144) and DHA (P = 0·388) (Table 2). Fig. 1 shows the levels of anti-oxidized-LDL antibodies and EPA according to age. The increase in levels of anti-oxidized-LDL antibodies was related significantly (P < 0·001) to lower EPA levels.
Table 2 Multiple regression analysis with the overall sample where the dependent variable is level of anti-oxidized-LDL antibodies and independent variables are age, BMI, waist:hip ratio, cholesterol, TAG, HDL-cholesterol, LDL-cholesterol, myristic acid, arachidonic acid, oleic acid, linoleic acid, DHA, EPA and palmitic acid*
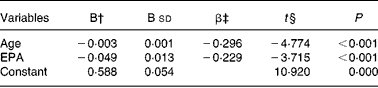
* For details of subjects and procedures, see Materials and methods.
† The unstandardized coefficients are the coefficients of the estimated regression model.
‡ The standardized coefficients or β are an attempt to make the regression coefficients more comparable.
§ t Statistics help you determine the relative importance of each variable in the model.

Fig. 1 Mean anti-oxidized-LDL antibodies (- - -) and EPA (—) for each 5-year age group in the overall study group. OD, optical density.
Discussion
The main finding of this study is that the variables which best explained the levels of anti-MDA-LDL antibodies in a group of healthy women were age and the percentage of EPA present in the serum phospholipids.
Earlier studies by our group showed that anti-oxidized-LDL antibodies have an inverse association with cholesterol concentrations(Reference Tinahones, Gomez-Zumaquero and Rojo-Martinez21) and that they fall markedly after the age of 35 years in the general population, with women having higher levels of anti-oxidized-LDL antibodies than men(Reference Tinahones, Gomez-Zumaquero and Garrido-Sanchez22). Other studies have also found that young persons have higher levels of these antibodies than older persons(Reference Karabinos, Koulouris and Melpidou10). Lower levels of anti-oxidized-LDL antibodies have also been reported in elderly persons with a high cardiovascular risk(Reference Balada, Ordi-Ros, Matas, Mauri, Bujan and Vilardell-Tarres23).
The clinical importance of these antibodies is controversial. Unlike early results and those of other studies that found high levels of antibodies in patients with atherosclerosis(Reference Lehtimaki, Lehtinen and Solakivi11), other results support the finding of an inverse association between the level of these autoantibodies and the presence of atherosclerosis(Reference Festa, Kopp, Schernthaner and Menzel24, Reference Karvonen, Paivansalo, Kesaniemi and Horkko25). Various studies support the idea that these antibodies impede the uptake of oxidized LDL by macrophages in the vessel wall and conversion in the foamy cells(Reference Horkko, Bird and Miller26). Indeed, the induction of these antibodies in experimental models slows the atherosclerotic process(Reference Freigang, Horkko, Miller, Witztum and Palinski27).
The results of this study show an inverse relation between the serum phospholipid levels of anti-oxidized-LDL antibodies and the serum phospholipid EPA (n-3 20:5) in the women studied. The possible association between the levels of anti-oxidized-LDL antibodies and EPA had not, as yet, been examined, although the possible relation of the n-3 PUFA with susceptibility of LDL to oxidation and the association with CVD has been studied(28). The fatty acid content of the LDL is a reflection of the dietary intake and may modify the susceptibility of the LDL to oxidation(Reference Reaven, Parthasarathy, Grasse, Miller, Steinberg and Witztum16).
Consumption of moderate amounts of fish oil has been shown to be beneficial in protecting against CHD(Reference Burr, Fehily and Gilbert29). Studies have shown how a diet rich in fish oil can affect different cardiovascular risk factors, such as lipid metabolism(Reference Schmidt, Kristensen, De Caterina and Illingworth30), platelet function(Reference Levine, Fisher and Schneider31), blood pressure(Reference Singer, Wirth and Berger32), blood viscosity(Reference Green, Fuchs and Schoenfeld33) and inflammatory processes(Reference Kinsella, Lokesh and Stone34). The beneficial effects have been attributed to the high levels of n-3 PUFA found in fish oil, especially EPA and DHA. Additionally, the oxidation of LDL may increase its atherogenicity, with the susceptibility of LDL to oxidative modification being one of the most important factors in its atherogenicity(Reference Witztum and Steinberg35).
Other studies have shown that enriching the LDL with n-3 PUFA increases the susceptibility of LDL to oxidation(Reference Tsai and Lu36, Reference Suzukawa, Ishikawa, Yoshida and Nakamura37). These studies show how the oxidation of LDL may be influenced by their content in PUFA (amount of substrate available for oxidation) and by their antioxidant content (conferring resistance to oxidation). Several studies that examined the effects of diet or fish oil supplements on the susceptibility of LDL to oxidation found a marked reduction in the lag phase of LDL oxidation(Reference Tsai and Lu36, Reference Suzukawa, Ishikawa, Yoshida and Nakamura37). However, not all studies agree with these findings(Reference Higdon, Du, Lee, Wu and Wander38) and it is not clear whether the two major n-3 PUFA in fish oil, EPA and DHA, are equally potent in decreasing the lag time for oxidation. EPA and DHA have recently been reported to have different effects on the susceptibility of LDL to oxidation. DHA does not appear to increase the susceptibility of LDL to oxidation to the same degree as EPA(Reference Mesa, Buckley, Marieinibane and Yaqoob39).
Thus, a paradoxical relation exists in our study; if the EPA increases LDL capacity for oxidation, the levels of anti-oxidized-LDL antibodies should rise. Different studies have shown that the levels of these antibodies have no direct association with the amount of oxidized LDL(Reference Nomura, Kanazawa and Fukuhara40). Proof of this poor relation between oxidized LDL and the levels of anti-oxidized-LDL antibodies is the lack of a direct relation between LDL and these antibodies; indeed an inverse relation has been reported in the general population(Reference Karabinos, Koulouris and Melpidou10, Reference Tinahones, Gomez-Zumaquero and Rojo-Martinez21). However, other studies have found a positive association between levels of anti-oxidized-LDL antibodies and modified LDL, suggesting that the levels of anti-oxidized-LDL antibodies reflect the in vivo oxidation of LDL(Reference Palinski, Rosenfeld and Yla-Herttuala8, Reference Craig9).
Our findings agree with a study in which EPA was given to hyperlipidaemic patients with type 2 diabetes(Reference Nomura, Kanazawa and Fukuhara40). This study showed how markers of platelet activation, E-selectin and anti-oxidized-LDL antibodies fell significantly, suggesting that the administration of EPA to these patients may prevent the development of complications caused by the oxidized LDL, E-selectin, or monocytes(Reference Nomura, Kanazawa and Fukuhara40). This same effect is produced by the consumption of fish oil. Its consumption can reduce levels of intercellular adhesion molecule-1 and P-selectin in patients with atherosclerosis(Reference Johansen, Seljeflot, Hostmark and Arnesen41), and produce a reduction in the up-regulation of other inflammatory markers during atherosclerosis(Reference Thies, Miles and Nebe-von-Caron42). Clinical and experimental studies show an anti-atherosclerotic effect of a diet rich in fish oil(Reference Aguilera, Ramirez-Tortosa, Mesa, Ramirez-Tortosa and Gil43, Reference Hu, Bronner and Willett44). Although the mechanism of inhibition of atherosclerosis by fish oil remains unclear, this anti-atherosclerotic effect may be related with the modulation of lipid metabolism(Reference Harris45), the improvement of vascular endothelial function(Reference Mesa, Buckley, Marieinibane and Yaqoob39), the reduction in cytokine production(Reference Alexander46) and the inhibition of the inflammatory process(Reference Simopoulos47). The anti-inflammatory effect of EPA is probably one of the causes of the inverse relation found in our study between EPA and the levels of anti-oxidized-LDL antibodies.
One of the limitations of this study is that it could not determine the fatty acid composition of LDL phospholipids, which confer the LDL with susceptibility to oxidation. Nevertheless, as LDL is the most common lipoprotein in blood, measurement of the fatty acid composition of the serum phospholipids may reflect the fatty acid composition of the LDL phospholipids.
The results of our study, like those of others, indicate the presence of a relation between different fatty acids and certain mechanisms involved in the development or prevention of atherogenesis. This gives an idea of the importance of adopting a healthy diet for the correct prevention of CVD.
In conclusion, the results of this study show that the fatty acid composition of the serum phospholipids, and especially the percentage of EPA, are inversely related with the levels of anti-oxidized-LDL antibodies.
Acknowledgements
The research group belongs to the Physiopathology of Obesity and Nutrition CIBER (CB06/03/0018), CIBER Diabetes y Enfermedades metabólicas (CB07/08/0019) of the Instituto de Salud Carlos III and FIS G03/152. The authors declare that there was no conflict of interest associated with this study. We thank Ian Johnstone for the English language version of the text.





