Branched-chain amino acids (BCAA) are essential amino acids (leucine, isoleucine and valine). Their main sources are meat, fish, legumes, dairy products and eggs. An adequate intake of BCAA is required for protein synthesis and several metabolic and signalling functions, including insulin metabolism(Reference de la, Zazpe and Ruiz-Canela1,Reference Holecek2) .
Several studies suggested positive associations between dietary or plasma BCAA and the risk of various diseases, including insulin resistance, type 2 diabetes, obesity, CVD and pancreatic cancer(Reference de la, Zazpe and Ruiz-Canela1,Reference Katagiri, Goto and Nakagawa3,Reference Ananieva and Wilkinson4) , which may share aetiological aspects and mechanisms with colorectal cancer (CRC). There is evidence of an elevated risk of CRC with higher consumption of red meat(Reference Alexander, Weed and Miller5), the most major source of BCAA. A study based on three US cohorts (The Nurses’ Health Study I and II, the Health Professionals Follow-up Study) including a total of 3309 incident cases investigated the association between dietary BCAA and CRC risk, reporting no association with the intake of leucine, isoleucine and valine(Reference Katagiri, Song and Zhang6). However, it found inverse association for distal colon in the Health Professionals Follow-up Study and rectum in The Nurses’ Health Study. A case–control Japanese study on 629 cases of colorectal adenoma found that higher levels of plasma BCAA were inversely associated with adenoma risk, a precursor lesion of CRC, in men but not in women(Reference Budhathoki, Iwasaki and Yamaji7).
To provide information on the issue, this article investigates the relation between dietary BCAA and CRC with specific focus on distal subsites using data from a multicentric case–control study of CRC(Reference La Vecchia, Braga and Negri8).
Methods
A case–control study of CRC was conducted between January 1992 and June 1996 in six Italian areas(Reference La Vecchia, Braga and Negri8). Cases were subjects with incident, histologically confirmed CRC and no previous diagnoses of cancer. They included 1953 subjects with cancer of the colon-rectum (1125 men and 828 women, median age 62, range 19–79, years) according to the International Classification of Diseases, 9th edition (ICD-9). Of these, 185 were grouped into the right colon, including the caecum, ascending colon and hepatic flexure (ICD-9 153.0, 153.4, 153.5 and 153.6), 188 into the transverse and descending colon (ICD-9 153.1, 153.2 and 153.7), 442 into the sigmoid colon (ICD-9 153.3) and 728 into the rectum, including the rectosigmoid junction (ICD-9 154.0–154.1). The remaining 410 cases belonged to other or unspecified anatomic subsites. One hundred forty-four cases (7·4 %) had a family history of CRC in first-degree relatives; 26 (1·3 %) received a previous diagnosis of intestinal adenomas.
Controls were patients with no history of cancer admitted to major teaching and general hospitals in the same catchment areas of cases for acute, non-neoplastic conditions, unrelated to hormonal or digestive tract diseases or to long-term modifications of diet. They included 2073 men and 2081 women aged 19–74 years (median age 58 years) from the following diagnostic categories: traumas (27 %) and other orthopaedic disorders (24 %), acute surgical conditions (18 %), eye diseases and other miscellaneous diseases (31 %). Seventy-six controls (1·8 %) had a family history of CRC in first-degree relatives; 39 (0·9 %) received a diagnosis of intestinal adenomas. There was no difference in terms of adenomas between cases and controls (P for χ 2 test, 1·63). On average, about 4 % cases and controls, when invited, refused to participate in the study.
A structured questionnaire was used to collect data on socio-demographic characteristics, such as education and occupation, lifetime smoking and alcohol-drinking habits, physical activity, anthropometric measures, personal medical history and cancer family history.
A reproducible(Reference Franceschi, Negri and Salvini9) and valid(Reference Decarli, Franceschi and Ferraroni10) FFQ was used to assess usual diet, including questions on the average weekly consumption of seventy-eight foods, food groups or recipes, and of five alcoholic beverages. From these data, we obtained the intakes of energy and selected nutrients, including leucine, isoleucine, valine and Ca, using an Italian food composition database, appropriately integrated with other data when needed(Reference La Vecchia, Braga and Negri8,Reference Salvini, Parpinel and Gnagnarella11) .
Given the high collinearity between the intakes of leucine, isoleucine and valine (r ~ 1·00), we focused on the analyses of total BCAA instead of single BCAA intakes. We categorised BCAA intakes into quintiles (based on the distribution of controls) and estimated the OR and the 95 % corresponding CI through multiple logistic regression models. The core model included terms for sex, age (quintiles; categorically), study centre (categorically), education (<7, 7–11 and ≥12 years; categorically), occupational physical activity (low, medium and high; categorically), BMI (quintiles; categorically), alcohol consumption (quartiles; categorically), tobacco smoking (never, former, <15 and ≥15 cigarettes/d current smokers; categorically), family history of CRC (yes/no), aspirin use (yes/no), menopausal status and postmenopausal hormone use (premenopause, never and ever users in postmenopause; in women only, categorically) and total energy intake (quintiles; categorically).
BCAA intake was also entered as continuous variables for an increment of the difference between the fourth and first quintile upper cut points as a measure of variability in the data. Further models also included one term for the intake of protein, fibre, folate, vitamin D, Ca and various BCAA sources (red meat, chicken and poultry, fish and dairy products) at a time (quintiles; categorically).
Results
Table 1 gives the means and standard deviations of BCAA intake, age, BMI, and selected nutrient intakes and food consumptions according to the BCAA quintiles among controls. The distribution of potential confounders was reported across BCAA quintiles. Participants with higher BCAA intake were younger and more frequently women. They were more frequently alcohol drinkers and current smokers. Women with higher BCAA intake were more likely to be in postmenopause. Participants with higher BCAA intake reported a higher intakes of energy, proteins, fibre, folate, vitamin D and Ca.
Table 1. Distribution of potential confounders by quintiles of branched-chain amino acid (BCAA) intake among 4154 controls, Italy, 1992–1996 (Mean values and standard deviations; percentages)
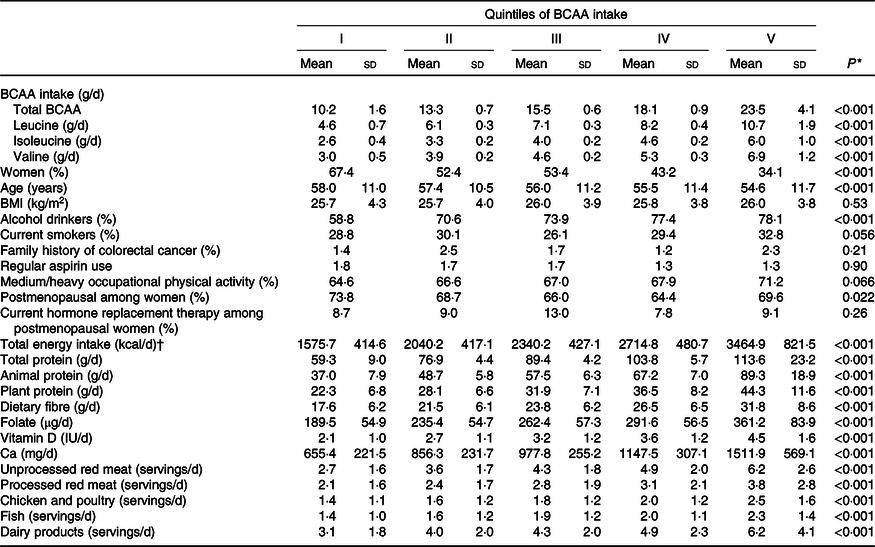
* For continuous variables: one-way ANOVA; for categorical variables: χ 2 1 test.
† To convert kcal to kJ, multiply by 4·184.
Table 2 shows the mean intake of total BCAA (16·1 g/d) and their quintile upper cut points (12·1, 14·5, 16·7 and 19·8 g/d) among controls. Multiple logistic regression OR of BCAA intake and their corresponding 95 % CI were given according to quintile (with the first quintile as a reference category) as well as continuous increment of intake, for all CRC and by anatomic subsites, from two major confounder adjusted models which differ by the addition of Ca intake.
Table 2. Multiple logistic regression-derived OR and corresponding 95 % CI according to quintile of branched-chain amino acid (BCAA) intake among 1953 cases with colorectal cancer and 4154 controls overall and by anatomic subsites, Italy, 1992–1996 (Mean values and standard deviations; odds ratios and 95 % confidence intervals)
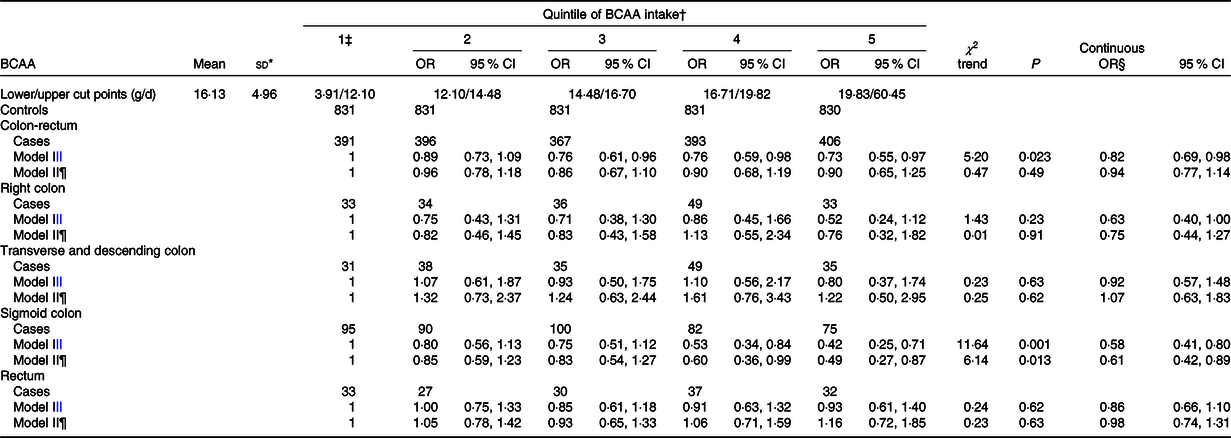
* Mean intake and standard deviation among controls.
† Control generated quintiles.
‡ Reference category.
§ Estimated for an increment equal to the difference between the fourth and the first cut-off quintile.
|| Adjusted for sex, age, study centre, education, occupational physical activity, BMI, alcohol consumption, tobacco smoking, family history of colorectal cancer, aspirin use, menopausal status and postmenopausal hormone use, and total energy intake.
¶ Further adjusted for Ca intake.
We observed an inverse association between BCAA intake and CRC risk (OR for the highest v. the lowest quintile 0·73; 95 % CI 0·55, 0·97; P for trend, 0·023) that however disappeared after adjustment of Ca intake (OR 0·90; 95 % CI 0·65, 1·25; P for trend, 0·49). The continuous OR was 0·82 (95 % CI 0·69, 0·98) in the first and 0·94 (95 % CI 0·77, 1·14) in the second model. Analysing separately anatomic subsites, we observed a linear inverse association for sigmoid colon (OR for the highest v. the lowest quintile 0·42; 95 % CI 0·25, 0·72; P for trend, 0·001) which remained after the adjustment for Ca (OR 0·49; 95 % CI 0·27, 0·87; P for trend, 0·013), with continuous OR about 0·60 in both models. No association was found for other subsites.
Table 3 gives the mean intake of leucine (7·3 g/d), isoleucine (4·1 g/d) and valine (4·7 g/d), as well as the logistic regression OR for their quintile and for a continuous increment of intake overall according to the two adjusted models. The OR for the three single BCAA intakes were almost identical to the OR for total BCAA.
Table 3. Multiple logistic regression-derived OR and corresponding 95 % CI according to quintile of leucine, isoleucine and valine intakes among 1953 cases with colorectal cancer and 4154, Italy, 1992–1996 (Mean values and standard deviations; odds ratios and 95 % confidence intervals)
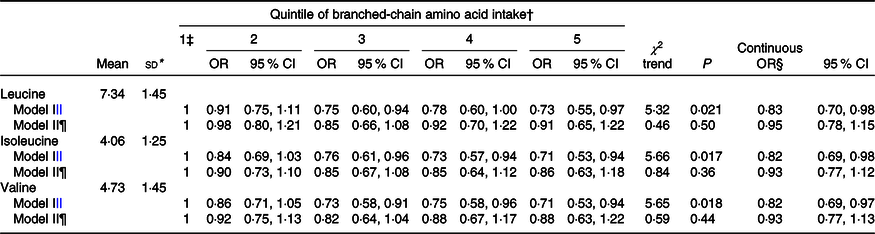
* Mean intake and standard deviation among controls.
† Control generated quintiles.
‡ Reference category.
§ Estimated for an increment equal to the difference between the fourth and the first cut-off quintile.
|| Adjusted for sex, age, study centre, education, occupational physical activity, BMI, alcohol consumption, tobacco smoking, family history of colorectal cancer, aspirin use, menopausal status and postmenopausal hormone use, and total energy intake.
¶ Further adjusted for Ca intake.
The estimates did not appreciably differ when considering further adjustment for selected measures of dietary quality and BCAA sources (Table 4).
Table 4. Multiple logistic regression-derived OR and corresponding 95 % CI according to quintile of branched-chain amino acid (BCAA) intake among 1953 cases with colorectal cancer and 4154 controls after adjustment of dietary factors, Italy, 1992–1996 (Odds ratios and 95 % confidence intervals)
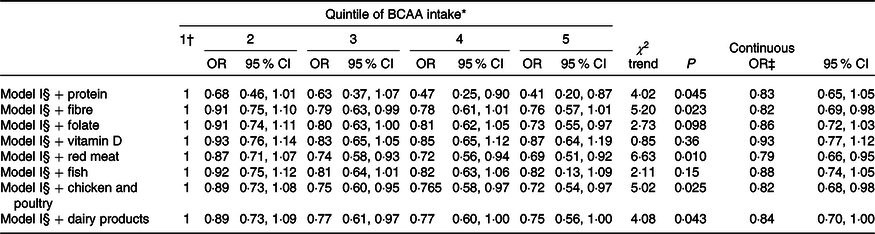
* Control generated quintiles.
† Reference category.
‡ Estimated for an increment equal to the difference between the fourth and the first cut-off quintile.
§ Adjusted for sex, age, study centre, education, occupational physical activity, BMI, alcohol consumption, tobacco smoking, family history of colorectal cancer, aspirin use, menopausal status and postmenopausal hormone use, and total energy intake.
Discussion
In this large case–control study, we found an inverse association between dietary BCAA (leucine, isoleucine and valine) and CRC risk in multivariable models, which, however, was not confirmed after adjustment for Ca intake. When separately analysing CRC sites, we observed that BCAA intake was inversely associated with sigmoid colon cancer risk also after adjustment for other dietary factors, including Ca intake.
Our data reinforce previous results from three large USA cohorts (the Nurses’ Health Study I and II, and the Health Professionals Follow-up Study) that did not support the hypothesis of a positive association between dietary BCAA and CRC risk(Reference Katagiri, Song and Zhang6), but suggested an inverse association for distal colon cancer, also after adjustment of Ca intake, as well as for rectal cancer(Reference Katagiri, Song and Zhang6). In the Nurses’ Health Study and Health Professionals Follow-up Study cohorts, similar differential associations for distal cancer as compared with other colorectal subsites were observed for processed and unprocessed meat intake, too(Reference Bernstein, Song and Zhang12). The absence of a clear mechanistic or biological explanation for different effects of BCAA on CRC risk by location leaves however the interpretation open to further investigations and discussion. The BCAA metabolism and BCAT1 activity (enzyme involved in the first step of BCAA catabolism) could play functional roles in the progression of tumours(Reference Ananieva and Wilkinson4). Increased levels of plasma BCAA were associated with an increased risk of pancreatic cancer in a nested case–control study of a Japan cohort(Reference Katagiri, Goto and Nakagawa3). They were also positively related to obesity, diabetes and insulin resistance(Reference Wang, Larson and Vasan13–Reference Zheng, Li and Qi15), which are known risk factors for CRC(Reference Rosato, Tavani and Gracia-Lavedan16,Reference Giovannucci17) . BCAA up-regulate glucose transporters and activate insulin secretion(Reference Holecek2). High BCAA levels activate the mammalian target of rapamycin complex 1 that could be linked to insulin resistance(Reference de la, Zazpe and Ruiz-Canela1) and elevated levels of blood insulin could cause alteration in the insulin-like growth factor, which is involved in the development of CRC(Reference Masahito, Yohei and Junpei18).
BCAA supplementation in mice with obesity and hyperinsulinaemia was found to improve insulin resistance and to inhibit the activation of the insulin-like growth factor/insulin-like growth factor-I receptor axis, thereby preventing the development of colonic premalignancies in an obesity-related colon cancer model(Reference Shimizu, Shirakami and Iwasa19). However, only a few studies, on circulating BCAA levels tend to support the hypothesis of an inverse association between BCAA and CRC risk, with unconvincing results. In particular, a metabolomics study found a significant reduction in terms of leucine and valine serum concentrations intake in sixty-four CRC cases as compared with sixty-five controls. This difference, however, was not confirmed when another MS instrument was used(Reference Qiu, Cai and Su20). Moreover, a case–control study from Japan reported that plasma BCAA concentration was inversely associated with the risk of colorectal adenoma in 629 cases and 584 controls(Reference Budhathoki, Iwasaki and Yamaji7). However, besides the difference in the outcome and the limitations due to a cross-sectional design, there is low agreement between dietary and plasma BCAA levels(Reference Iwasaki, Ishihara and Takachi21) which may explain the difference with null results, observed in our and previous findings on dietary BCAA. Plasma BCAA can be interpreted as a marker of a metabolism alteration of BCAA levels related to insulin resistance(Reference Lynch and Adams22).
Major sources of BCAA intake in our data were red meat (26 %), dairy products (13 %), poultry (12 %) and fish (7 %). Most models further adjusted for these food groups are clearly overadjusted, but did not alter the results, as did the inclusion of other nutrients in the models. In particular, no appreciable effect modification was observed in the model including dairy products, whose consumption is partially correlated with Ca intake. Only the adjustment for fish consumption and vitamin D slightly increased the OR estimates (0·82 and 0·87 for the highest v. the lowest quintile of BCAA intake, respectively), suggesting a possible role of vitamin D, in addition to Ca, in explaining the observed associations with CRC, in line with the literature(Reference Lipworth, Bender and Rossi23–Reference Huncharek, Muscat and Kupelnick26). In an additional model, including both dietary Ca and vitamin D, no association with BCAA was evident.
This study was sufficiently large to obtain precise risk estimates for BCAA intake. Cases and controls came from comparable catchment areas, participation was virtually complete reducing potential selection bias. Moreover, the hospital setting is unlikely to have reduced the comparability of diet recall by cases and controls(Reference D’Avanzo, La Vecchia and Katsouyanni27), and the estimate of BCAA intake derives from a satisfactorily reproducible and valid FFQ(Reference Franceschi, Negri and Salvini9,Reference Decarli, Franceschi and Ferraroni10) . A limitation of this study is the lack of plasma samples to assess the circulating BCAA levels and to compare variability and risk estimates with results from dietary BCAA. With reference to confounding, all OR were adjusted for age and other major confounding factors, including education, physical activity and total energy.
In conclusion, this study provides supporting evidence that higher levels of dietary BCAA intake are not associated with an increase of CRC risk, but may be related to a reduced risk of sigmoid colon cancer.
Acknowledgements
This work was supported by the Italian Foundation for Cancer Research (AIRC) (My First AIRC grant no. 17070).
M. R. and C. L. V. designed the research; M. R., F. M. and C. L. V. drafted the manuscript; M. R. and F. M. conducted the analysis; M. P. contributed to the design of the analysis and interpretation of results. D. S., A. G. and C. L. V. designed and carried out the initial study; D. S., A. C., E. C., A. G. and C. L. V. were involved in the collection of the data. All authors contributed to the critical revision and approval of the manuscript.
The authors declare that there are no conflicts of interest.







