Hydroxycitrate (HCA), a compound having a structure similar to citrate, is enriched in an Indian fruit known as Garcinia cambogia (Reference Lewis and Neelakantan1, Reference Tomita, Okuhara and Shigematsu2), which is commonly used as a traditional Indian medicine. It is generally known that citrate is an allosteric regulator for a number of enzymes that are involved in carbohydrate and fat metabolism, such as phosphofructokinase, a key enzyme regulating glycolysis(Reference Garland, Randle and Newsholme3), and acetyl Co-A carboxylase, a key enzyme regulating fatty acid synthesis(Reference Munday4). HCA can also serve as a competitive inhibitor for ATP-citrate lyase(Reference Watson, Fang and Lowenstein5), which directs energy metabolism towards fatty acid oxidation. Therefore, administration of HCA is expected to cause metabolic ramification in vivo. The results of several mouse studies support the idea that oral HCA supplementation lowered plasma insulin concentration(Reference Hayamizu, Hirakawa and Oikawa6), enhanced the whole-body fatty acid oxidation and spared muscle glycogen(Reference Ishihara, Oyaizu and Onuki7).
There is a paucity of information regarding the effects of HCA supplementation on fat metabolism in human subjects. Lim et al. (Reference Lim, Ryu and Nho8) have previously reported that short-term HCA supplementation significantly lowered respiratory quotient (RQ) during exercise. Reduced RQ reflects energy reliance towards fat metabolism. In contrast, Kriketos et al. performed a double-blind, randomised, crossover study involving 3-d supplementation of HCA or placebo for a group of male subjects. They found that RQ status was not significantly changed during rest or during the moderate intensity exercise compared with the placebo treatment. Whether HCA supplementation can lower increased energy reliance on fat oxidation during post-exercise recovery is currently unknown. Furthermore, HCA was also found to influence carbohydrate metabolism in animals fed with a high-glucose diet, evidenced by a decrease in the area under the curve after an oral glucose loading(Reference Leonhardt, Balkan and Langhans9). Yet, no published human data are available regarding the effect of HCA on insulin sensitivity and muscle glycogen synthesis after exercise.
Skeletal muscle is capable of using both carbohydrate and fat to supply energy for muscular contraction. In these tissues, transportation of glucose and fatty acid from the circulation across the sarcolemma relies on GLUT4 and FAT/CD36 transporters, respectively(Reference Luiken, Dyck and Han10). It is currently unknown whether HCA supplementation can alter the expression levels of both transmembrane transporter proteins in human skeletal muscle. The level of GLUT4 expression in skeletal muscle is directly associated with the rate of insulin-stimulated glycogen stores and whole-body insulin sensitivity(Reference Ivy and Kuo11). In general, when glucose transport process is impaired due to insulin resistance or insulin deficiency, the fatty acid transporter FAT/CD36 is up-regulated in skeletal muscle to compensate for the impaired carbohydrate utilization(Reference Bonen, Tandon and Glatz12, Reference Kahn, Rosen and Bak13).
In animal models, glycogen levels in skeletal muscle can be increased by HCA supplementation(Reference Ishihara, Oyaizu and Onuki7). Currently, there is no evidence whether this treatment can also increase glycogen stores in human skeletal muscle after exercise. In the present study, we hypothesised that HCA supplementation following 1 h of exercise can alter the rate of glycogen synthesis in human skeletal muscle corresponding to changes of GLUT4 and FAT/CD36 gene expression. Since skeletal muscle plays a major role in postprandial glucose uptake in human subjects, the whole-body insulin sensitivity and glucose tolerance were also measured in the present study.
Materials and methods
Ethics statement
This protocol was approved by the National Taiwan Sport University Ethics Committee and submitted to the International Standard Randomised Controlled Trial Number (ISRCTN45782014) for registration. The nature, purpose and possible risks of the study were explained to each subject before written consent was obtained. This study was conducted in accordance with the guidelines laid down in the Declaration of Helsinki.
Subjects
Vastus lateralis muscle biopsies were obtained from eight healthy male volunteers (age, 22·0 (se 0·3) years; height, 171·3 (se 0·7) cm; weight, 73·7 (se 1·1) kg; BMI, 25·2 (se 0·5) kg/m2; ![]() , 45·7 (se 1·5) ml/kg per min). All subjects were instructed to refrain from heavy physical activity and to consume the same diet for 2 d before each trial. All subjects were asked to abstain from alcohol, caffeine and tobacco consumption for 24 h before trials.
, 45·7 (se 1·5) ml/kg per min). All subjects were instructed to refrain from heavy physical activity and to consume the same diet for 2 d before each trial. All subjects were asked to abstain from alcohol, caffeine and tobacco consumption for 24 h before trials.
Experimental procedure
Maximal oxygen consumption (![]() ) of all the subjects was measured 2 d before the exercise trial. On the day of the trial, the subjects reported to the laboratory at 08.00 hours after an overnight fast. They warmed up for 5 min and performed a 60-min cycling exercise at a 75 %
) of all the subjects was measured 2 d before the exercise trial. On the day of the trial, the subjects reported to the laboratory at 08.00 hours after an overnight fast. They warmed up for 5 min and performed a 60-min cycling exercise at a 75 % ![]() while drinking water was available ad libitum during and after the exercise(Reference Cheng, Liao and Liu14). The trial order for the eight subjects was randomised so that four subjects started with the HCA trial, while four subjects started with the placebo trial. The crossover trial was repeated after completion of the first trial with a 1-week washout period. Since we aimed to determine the effect of HCA on recovery, HCA and placebo meals were provided to the subjects immediately after exercise. The meal contained 70 % carbohydrate with 500 mg HCA in 0·5 litres drinking water for a 3-h recovery, whereas the placebo trial (placebo, eight comparisons) provided a diet containing 70 % carbohydrate content and the same volume of drinking water. In particular, the carbohydrate meal contained 2 g carbohydrate per kg of body weight. The decision for HCA dosage was based on a previous human report that demonstrated a positive ergogenic effect on endurance(Reference Tomita, Okuhara and Shigematsu2). The carbohydrate meal consisted of Corn Flakes™ (Kellogg's (UK) Limited, Manchester, UK), skimmed milk, white bread, strawberry jam and water, with an overall glycaemic index of 76·6. The average energy intake of the diet for the all subjects was 2880·7 (se 82·8) kJ (carbohydrate, 140·1 (se 3·9) g; protein, 19·7 (se 0·6) g; fat, 5·5 (se 0·1) g). The meal was consumed within 10 min. HCA was purchased from Yuluen Limited (Taipei, Taiwan). Muscle biopsy samples were obtained immediately after exercise and 3 h after exercise for determination of muscle glycogen, GLUT4 and FAT/CD36 expressions. Glucose, insulin, NEFA and glycerol levels were also measured during the 3-h post-exercise recovery.
while drinking water was available ad libitum during and after the exercise(Reference Cheng, Liao and Liu14). The trial order for the eight subjects was randomised so that four subjects started with the HCA trial, while four subjects started with the placebo trial. The crossover trial was repeated after completion of the first trial with a 1-week washout period. Since we aimed to determine the effect of HCA on recovery, HCA and placebo meals were provided to the subjects immediately after exercise. The meal contained 70 % carbohydrate with 500 mg HCA in 0·5 litres drinking water for a 3-h recovery, whereas the placebo trial (placebo, eight comparisons) provided a diet containing 70 % carbohydrate content and the same volume of drinking water. In particular, the carbohydrate meal contained 2 g carbohydrate per kg of body weight. The decision for HCA dosage was based on a previous human report that demonstrated a positive ergogenic effect on endurance(Reference Tomita, Okuhara and Shigematsu2). The carbohydrate meal consisted of Corn Flakes™ (Kellogg's (UK) Limited, Manchester, UK), skimmed milk, white bread, strawberry jam and water, with an overall glycaemic index of 76·6. The average energy intake of the diet for the all subjects was 2880·7 (se 82·8) kJ (carbohydrate, 140·1 (se 3·9) g; protein, 19·7 (se 0·6) g; fat, 5·5 (se 0·1) g). The meal was consumed within 10 min. HCA was purchased from Yuluen Limited (Taipei, Taiwan). Muscle biopsy samples were obtained immediately after exercise and 3 h after exercise for determination of muscle glycogen, GLUT4 and FAT/CD36 expressions. Glucose, insulin, NEFA and glycerol levels were also measured during the 3-h post-exercise recovery.
Muscle sample collection
Muscle biopsies were performed under local anaesthesia (2 % lidocaine without adrenaline). Using an aseptic technique, we made a 10 mm long and 10 mm deep incision in the skin and muscle fascia at about 20 cm above the knee. Biopsies were obtained from the right vastus lateralis muscle (about 50 mg) using the Bergstrom(Reference Bergstrom15) percutaneous biopsy technique. Samples were blotted dry and grossly dissected free of fat and connective tissue, frozen in liquid N2 and stored at − 80°C before analyses of muscle glycogen, GLUT4 and FAT/CD36 expressions.
Glycogen assay
Approximately 25 mg skeletal muscle from the deep portion of the vastus lateralis was dissolved in 1 m-KOH at 75°C for 30 min. Dissolved homogenate was neutralised by glacial acetic acid and incubated overnight in acetate buffer (0·3 m-sodium acetate, pH 4·8) containing amyloglucosidase (Boehringer Mannheim, Indianapolis, IN, USA). The reaction mixture was neutralised with 1 m-NaOH. Samples were then analysed by measuring glucosyl units by the Trinder reaction (Sigma, St Louis, MO, USA).
Blood analyses
A 20-G polyethylene catheter (Jelco, Tampa, FL, USA) was placed in an antecubital vein for blood samples. Blood samples were then taken before and after meal consumption. Following each sample collection, the catheter was kept patent by flushing with a small amount of saline solution containing heparin (10 IU/ml). During recovery, blood samples were collected every 30 min for 180 min. Blood samples were collected into fluoride heparin and plasma tubes. Plasma was obtained after centrifuging at 4°C for 10 min at 3000 rpm and was stored at − 80°C before analysis. Blood glucose was determined by an automated glucose analyser (YSI Life Sciences, Yellow Springs, OH, USA). Plasma insulin levels were determined by using the RIA method with a commercial kit (Baylor Diagnostics, Tarrytown, NY, USA) according to the manufacturer's instruction. Blood glycerol was determined by a fluorometric method(Reference Laurell and Tibbling16). Plasma samples were also used for NEFA analysis (Wako, Neuss, Germany) with an automatic photometric analyzer (Roche, Basel, Switzerland).
Expired gas analysis
Samples of expired gas were analysed every 60 min using MetaMax3B (Cortex Biophysik, Leipzig, Germany) during the post-exercise recovery period (60, 120 and 180 min). ![]() and
and ![]() data were obtained from average values of six recording sessions, each of 10-min period.
data were obtained from average values of six recording sessions, each of 10-min period.
RT-PCR
Total RNA from the vastus lateralis muscle samples was extracted using TRI Reagent (Molecular Research Center, Cincinnati, OH, USA), followed by RT-PCR. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene template was used as an internal standard to quantify GLUT4 and FAT/CD36 mRNA expression. For amplification reaction, the oligonucleotide primer sequences of GLUT4, FAT/CD36 and GAPDH genes were selected by using the software Primer Select (DNASTAR, Madison, WI, USA) and were synthesised by MDBio (Taipei, Taiwan, ROC). The primer sequences for the GLUT4 gene were 5′-GGGGCCCTACGTCTTCCTTCT-3′ (forward) and 5′-CACGGCCAAACCACAACACAT-3′ (reverse). The primer sequences for FAT/CD36 were 5′-CTCTTTCCTGCAGCCCAATG-3′ (forward) and 5′-TCGCAGTGACTTTCCCAATA-3′ (reverse). The primer sequences for GAPDH gene were 5′-GAGTCAACGGATTTGGTCGT-3′ (forward) and 5′-GTTGTCATGGATGACCTTGG-3′ (reverse). A PCR master mix, containing 4 mm-MgCl2, 2·5 U of Taq polymerase and 6 pmol forward and reverse primers, was added to the newly synthesised complementary DNA samples to a total volume of 50 μl. The reactions for PCR amplification were heated to 94°C for 3 min and were then followed by a re-annealing step at 55°C. The elongation step was performed at 72°C for 60 s. The denaturing–annealing–elongation cycle was repeated thirty-two times. A 5-min elongation step at 72°C was carried out after the last cycle. The amplified PCR products of the internal standard and target mRNA were separated by 2·5 % NuSieve/agarose (3:1, w/w) gel electrophoresis and visualised by ethidium bromide staining. Gels were then photographed and quantified by densitometric analysis with a ZERO-Dscan (Scanalytics, Inc., FairFax, VA, USA). The densitometry analysis of each band was performed by scanning the gel with Scion Image (ZERO Dscan System; Scanalytics, Inc.) and the illumination was expressed as integrated optical density and normalised with the GAPDH as an internal control.
Western blotting
Muscles were homogenised in 20 mm-ice-cold HEPES, 1 mm-EDTA and 250 mm-sucrose buffer (pH 7·4) with a Polytron (Brinkmann Instruments, Westbury, NY, USA). Protein contents in each sample were quantified by the Lowry assay. Equal amounts of proteins were denatured and separated on 7·5 % SDS-PAGE and then transferred to poly(vinylidene difluoride) membranes (New Life Science Product, Inc., Boston, MA, USA). Non-specific binding sites on the membranes were blocked with 5 % non-fat dry milk in a buffer containing 10 mm-Tris-HCl and 100 mm-NaCl (pH 7·5), at 4°C overnight. The blots were incubated sequentially with GLUT4 (1:4000; Chemicon, Ramona, CA, USA), FAT/CD36 (1:150; Abcam, Cambridge, MA, USA) and β-actin primary antibodies (1:5000; Sigma) and horseradish peroxidase-conjugated antibodies (1:10,000; Cell Signaling, Danvers, MA, USA) after stripping procedures. Protein levels for GLUT4 and FAT/CD36 are expressed relative to β-actin from the same gel. Antigen–antibody complexes were visualised, detected and quantified with the ECL Western blot detection kit (Amersham Pharmacia Biotech, Inc., Piscataway, NJ, USA), Luminescent Image Analyzer (Fujifilm, Tokyo, Japan) and Zero-Dscan densitometric (Scanalytics, Inc., Rockville, MD, USA), respectively.
Statistical analysis
Differences between all measured variables were compared by using two-way repeated-measures ANOVA. Fisher's post hoc test, which holds the value of a type I error to 0·05 for each test, was used to distinguish significant differences between pairs of conditions. Data are presented as the mean and the standard error.
Results
HCA supplementation significantly increased energy reliance on fat oxidation, based on gaseous exchange measurement. Fig. 1(a) shows the respiratory exchange ratio results during 3-h post-exercise recovery for the two experimental trials (placebo: high-carbohydrate meal only; HCA: high-carbohydrate meal containing HCA; Fig. 1(a)).

Fig. 1 (a) Respiratory quotient, (b) plasma glucose, (c) insulin, (d) NEFA and (e) glycerol concentrations after post-exercise hydroxycitrate (–●–) supplementation. Values are means, with their standard errors, n 8. * Mean values were significantly different compared with placebo (–○–; P < 0·05). † Mean values were significantly different compared with values at 60 min post-exercise (P < 0·05). For insulin, 1 μU/ml = 6·945 pmol/l.
Plasma glucose and insulin levels during a 3-h post-exercise recovery meal are shown in Fig. 1(b) and (c), respectively. Both glucose and insulin concentrations peaked at 30 min following exercise and declined thereafter in the two trials. No significant difference in post-meal glucose was found between placebo and HCA trials. HCA supplementation significantly lowered post-meal insulin curve compared to the placebo trial (P < 0·05) (areas under the curve: HCA, 4148 (se 340); placebo; 6849 (se 429)). Glycogen data are given in Table 1. The glycogen synthesis rate of vastus lateralis in 3 h was onefold greater in the exercised subjects in the HCA trial than those in the placebo trial (P < 0·05). No difference was found in plasma NEFA and glycerol levels between the placebo and HCA trials (Fig. 1(d) and (e)). GLUT4 protein level after 3 h in the HCA trial was significantly lower than that in immediately post-exercise (0 h) and below placebo levels (Table 1), whereas FAT/CD36 mRNA after 3 h in HCA trial was significantly higher than that in immediately post-exercise (0 h) and above placebo levels (Table 1). Representative autoradiographs of GLUT4 and FAT/CD36 expression (mRNA and protein levels) are shown in Fig. 2.
Table 1 Glycogen content and substrate transporter gene expression profile in vastus lateralis muscle after post-exercise hydroxycitrate (HCA) supplementation*
(Mean values with their standard errors)
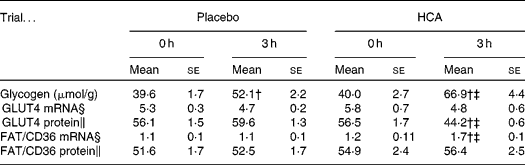
* Eight comparisons were performed on Western blotting.
† Mean values were significantly different compared with 0 h (immediately post exercise; P < 0·05).
‡ Mean values were significantly different compared with placebo trial (P < 0·05).
§ RNA levels for GLUT4 and FAT/CD3 are expressed relative to glyceraldehyde 3-phosphate dehydrogenase mRNA.
∥ Protein levels for GLUT4 and FAT/CD36 are expressed relative to β-actin.

Fig. 2 Representative autoradiographs of substrate transporter gene expression in vastus lateralis. (a) mRNA levels for GLUT4, FAT/CD36 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (b) Protein levels for GLUT4, FAT/CD36 and β-actin. HCA, hydroxycitrate.
Discussion
HCA has a structure similar to citrate(Reference Soni, Burdock and Preuss17), which is known as an allosteric regulator for enzymes controlling glycolysis and fatty acid metabolism(Reference Garland, Randle and Newsholme3–Reference Watson, Fang and Lowenstein5). In the present study, we tested the hypothesis that oral HCA supplementation can increase muscle glycogen stores and insulin sensitivity after exercise in human subjects, based on the evidence from studies carried out in mice(Reference Hayamizu, Hirakawa and Oikawa6, Reference Ishihara, Oyaizu and Onuki7). The main findings of the study are: (1) oral HCA supplementation significantly accelerated the rate of glycogen synthesis to twofold in exercised human skeletal muscle compared to placebo; (2) this increase occurred in parallel with increased whole-body insulin sensitivity, evidenced by reduced post-meal insulin response; (3) with greater amount of glycogen accumulation, GLUT4 protein was down-regulated but FAT/CD36 mRNA was up-regulated by HCA supplementation in skeletal muscle. The results of the study suggest that the greater glycogen synthesis rate after HCA supplementation is mediated by either reduced carbohydrate utilisation or increased fat utilisation (glycogen-sparing effect) in skeletal muscle.
Skeletal muscle is the main tissue responsible for postprandial glucose disposal from circulation, and thus the insulin sensitivity of skeletal muscle plays a major role in the whole-body insulin sensitivity. In the present study, improvement in the whole-body insulin sensitivity by HCA supplementation fits well with the outcome of enhanced glycogen synthesis rate in skeletal muscle. It has been shown in sedentary animals that oral HCA supplementation significantly lowers insulin response after glucose intake(Reference Hayamizu, Hirakawa and Oikawa6) and improves insulin sensitivity in skeletal muscle(Reference Richter, Derave and Wojtaszewski18). In line with this evidence, the present study extends the current knowledge to understand how HCA can improve whole-body insulin sensitivity in human subjects with aerobic exercise training.
In exercised skeletal muscle, increased glycogen synthesis by HCA supplementation appears to be associated, in part, with increased energy reliance on fat oxidation. Here, we found that HCA supplementation reduced RQ and increased estimated fat oxidation rate during the post-exercise recovery period. An animal study conducted previously by Ishihara et al. (Reference Ishihara, Oyaizu and Onuki7) has demonstrated both the glycogen-sparing effect and the increased fat oxidation with HCA supplementation at rest and during exercise. Different from the present study design, most of the previous studies determining the effect of HCA supplementation on fat oxidation were performed under sedentary conditions and during exercise(Reference Tomita, Okuhara and Shigematsu2, Reference Lim, Ryu and Nho8).
Not all the literature agrees with the finding that HCA supplementation can increase fatty acid oxidation(Reference Kriketos, Thompson and Greene19, Reference van Loon, van Rooijen and Niesen20). Despite using a much larger dose of HCA compared to the present study, Kriketos et al. (Reference Kriketos, Thompson and Greene19) and van Loon et al. (Reference van Loon, van Rooijen and Niesen20) observed no effect of HCA supplementation on fatty acid oxidation in human subjects. Their studies were conducted under fasting and moderate exercise conditions, whereas the present study performed the same measures during the post-exercise recovery period. Furthermore, RQ measurements in both the previous studies were performed < 2 h after HCA supplementation. Thus, such discrepancy may also be related to the fact that HCA delays intestinal absorption(Reference Wielinga, Wachters-Hagedoorn and Bouter21). This could explain the present study result that the HCA supplementation effect on lowering RQ was not evident until 2 h after the meal.
An alternative mechanism that might explain the HCA effect on increased rate of glycogen synthesis is its inhibitory effect on the glycolytic pathway. HCA has been found to inhibit phosphofructokinase, a key enzyme controlling glycolysis(Reference McCune, Foe and Kemp22). Once glucose enters intracellular space across sarcolemma, it is rapidly phosphorylated into glucose 6-phosphate and converted into glycogen through the glycogen synthesis pathway and lactate through the glycolytic pathway. Therefore, inhibition of the glycolysis pathway by HCA will divert more glucose 6-phosphate into the glycogen synthesis pathway in skeletal muscle. This condition would undoubtedly alter the energy dependence of exercised skeletal muscle on fatty acid relative to glucose in parallel with increased glycogen synthesis.
To the best of our knowledge, this is the first study that demonstrates an increased FAT/CD36 gene expression of exercised human skeletal muscle after HCA supplementation. The expression level of fatty acid transporter protein normally increases when intracellular glucose availability is reduced. Under this condition, the normal rate of ATP synthesis must be maintained by increasing the rate of fatty acid transport and oxidation. For example, FAT/CD36 expression in skeletal muscle can be increased by streptozotocin injection(Reference Luiken, Arumugam and Bell23), a treatment that eliminates insulin production from pancreatic β-cells, and thereby preventing the circulating glucose entry into muscle cells. FAT/CD36 protein level in skeletal muscle is higher in patients with both type 1 and type 2 diabetes(Reference Luiken, Arumugam and Bell23, Reference Bonen, Parolin and Steinberg24). The skeletal muscle of diabetes patients has impaired glucose transport from the circulation compared to normal people due to either insulin deficiency or insulin resistance. Increasing dietary fat availability can also increase FAT/CD36 expression in human skeletal muscle(Reference Cameron-Smith, Burke and Angus25). Conversely, glucose supplementation rapidly down-regulates FAT/CD36 expression in exercised skeletal muscle(Reference Civitarese, Hesselink and Russell26). Therefore, the present study result suggests a possibility that HCA supplementation causes skeletal muscle adaptation favouring fatty acid utilisation aimed to compensate the energy deficit because of inhibited glycolysis.
Another finding of the study is that HCA supplementation significantly suppressed muscle GLUT4 protein levels after exercise. This phenomenon may be associated with greater glycogen stores in skeletal muscle. A study carried out in rats previously has shown that carbohydrate supplementation significantly enhances the exercise training-induced GLUT4 protein expression in skeletal muscle until glycogen supercompensation(Reference Kuo, Browning and Ivy27). However, further increase in glycogen content after exercise can lead to down-regulation of GLUT4 protein expression in rat skeletal muscle(Reference Kuo, Hunt and Ding28). According to the above evidence, a negative feedback regulatory mechanism functions to keep muscle glycogen levels in a physiological range.
It is generally known that muscle glucose uptake and the whole-body insulin sensitivity are proportional to the level of GLUT4 protein in skeletal muscle(Reference Ivy and Kuo11). Yet in the present study we observed greater muscle glycogen accumulation with HCA supplementation with decreased GLUT4 protein level. This result points to a possibility that HCA supplementation elevates post-exercise GLUT4 protein translocation to plasma membrane, despite the total number being reduced. For the future, it would be more informative to measure the amount of each protein in human muscle that is located in the sarcolemma v. sequestered in intracellular stores. Furthermore, it is plausible to acknowledge that GLUT4 protein could be significantly reduced in only 3 h after HCA treatment. Regulation in GLUT4 protein number is responsive to exercise training, which can be balanced by controlling the rate of synthesis and degradation of the protein. We have previously shown the changes in GLUT4 that were evident in rodents 5–16 h after exercise(Reference Kuo, Browning and Ivy27). Regulation of GLUT4 protein stability has received less attention in the past. Compared to synthesis, degradation is more thermodynamically favourable for spontaneous process. Future investigation in this regard may offer insight into whether protein stability takes part in the regulation of fuel stores in human skeletal muscle.
Muscle glycogen is the main energy fuel for endurance in athletic competition. Normally, repletion of glycogen stores from exercise requires more than 1 d(Reference Ivy29). Interventions that can facilitate glycogen repletion would be helpful for optimising endurance performance on the next competition or next training session. In the present study, we demonstrate that HCA supplementation can effectively increase the rate of glycogen stores in skeletal muscle. The result of the study encourages further investigation for developing nutritional ergogenic aid for endurance athletes.
In a clinical aspect, the results of this study provide ground for investigating the use of HCA as an adjuvant supplement for exercise training in the treatment of type 2 diabetes. Exercise training is currently considered as an essential part of clinical intervention for type 2 diabetes patients. The present study provides new finding that HCA supplementation provides additional benefit to improve insulin sensitivity in human subjects. It would be promising to establish an adjuvent nutritional intervention that can enhance the exercise training effect in improving insulin sensitvity while increasing muscle glycogen stores, particularly for those insulin-resistant elderly with reduced physical fitness.
In the present study, no side effect was reported from all young participants. However, it should be noted that increasing fat oxidation after HCA supplementation could have a risk of increasing ketosis for patients with severe diabetes(Reference Foster and McGarry30). Prolonged excess of ketone bodies can overwhelm the bicarbonate buffering system in the bloodstream, leading to acidosis when blood pH falls to a certain extent. Therefore, potential harmful effects of HCA supplementation on diabetic patients still demand further evaluation.
In conclusion, the present study provides the first evidence that oral HCA supplementation can increase glycogen synthesis in exercised human skeletal muscle and lowers insulin response under oral glucose challenge. Furthermore, we found significant down-regulation of GLUT4 protein and up-regulation of FAT/CD36 mRNA in exercised skeletal muscle by oral HCA supplementation, in parallel with the increased whole-body fatty acid oxidation. The results of the present study suggest that HCA supplementation can be used to have an impact on metabolism in skeletal muscle, which, in turn, affects the whole-body insulin sensitivity.
Acknowledgements
This study was sponsored, in part, by a grant from the National Science Council (no. NSC 97-2140-H-166-007-MY2) and Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH100-TD-B-111-004). I.-S. C., S. D. L. and C.-H. K. contributed to experimental design and manuscript preparation. S.-W. H., H.-C. L., Y.-C. C., C.-L. W. and C.-Y. H. contributed to experimental design, technique support and muscle biopsy. There is no conflict of interest. This work was funded by the National Science Council and the National Sports Affair, Taipei, Taiwan.


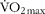 and received HCA or placebo in a crossover design repeated after a 7 d washout period. They consumed 500 mg HCA or placebo with a high-carbohydrate meal (2 g carbohydrate/kg body weight, 80 % carbohydrate, 8 % fat, 12 % protein) for a 3-h post-exercise recovery. Muscle biopsy samples were obtained from vastus lateralis immediately and 3 h after the exercise. We found that HCA supplementation significantly lowered post-meal insulin response with similar glucose level compared to placebo. The rate of glycogen synthesis with the HCA meal was approximately onefold higher than that with the placebo meal. In contrast, GLUT4 protein level after HCA supplementation was significantly decreased below the placebo level, whereas expression of fatty acid translocase (FAT)/CD36 mRNA was significantly increased above the placebo level. Furthermore, HCA supplementation significantly increased energy reliance on fat oxidation, estimated by the gaseous exchange method. However, no differences were found in circulating NEFA and glycerol levels with the HCA meal compared with the placebo meal. The present study reports the first evidence that HCA supplementation enhanced glycogen synthesis rate in exercised human skeletal muscle and improved post-meal insulin sensitivity.
and received HCA or placebo in a crossover design repeated after a 7 d washout period. They consumed 500 mg HCA or placebo with a high-carbohydrate meal (2 g carbohydrate/kg body weight, 80 % carbohydrate, 8 % fat, 12 % protein) for a 3-h post-exercise recovery. Muscle biopsy samples were obtained from vastus lateralis immediately and 3 h after the exercise. We found that HCA supplementation significantly lowered post-meal insulin response with similar glucose level compared to placebo. The rate of glycogen synthesis with the HCA meal was approximately onefold higher than that with the placebo meal. In contrast, GLUT4 protein level after HCA supplementation was significantly decreased below the placebo level, whereas expression of fatty acid translocase (FAT)/CD36 mRNA was significantly increased above the placebo level. Furthermore, HCA supplementation significantly increased energy reliance on fat oxidation, estimated by the gaseous exchange method. However, no differences were found in circulating NEFA and glycerol levels with the HCA meal compared with the placebo meal. The present study reports the first evidence that HCA supplementation enhanced glycogen synthesis rate in exercised human skeletal muscle and improved post-meal insulin sensitivity.


