Introduction
Shallow-water coral reefs are one of the most diverse ecosystems on Earth. Their structural framework provides a suitable habitat for diverse assemblages of organisms (Graham and Nash, Reference Graham and Nash2013). Bryozoans are a common and diverse component of the benthic cryptic faunas associated with these ecosystems (Winston and Jackson, Reference Winston and Jackson1984; Winston, Reference Winston1986; Cuffey, Reference Cuffey and Hopley2011; Di Martino and Taylor, Reference Di Martino and Taylor2014, Reference Di Martino and Taylor2015; Bastos et al., Reference Bastos, Moura, Moraes, Vieira, Braga, Ramalho, Amado-Filho, Magdalena and Webster2018; Ramalho et al., Reference Ramalho, Taylor, Moraes, Moura, Amado-Filho and Bastos2018). However, their roles in these ecosystems remain poorly known, and the species richness of the group is underestimated in most Recent and ancient reefs (Pearman et al., Reference Pearman, Leray, Villalobos, Machida, Berumen, Knowlton and Carvalho2018). Although extensive work was previously undertaken on fossil bryozoan collections from the Caribbean region (Cheetham et al., Reference Cheetham, Jackson, Sanner and Ventocilla1999; Cheetham and Jackson, Reference Cheetham, Jackson, Herrera-Cubilla and Jackson2000; Di Martino et al., Reference Di Martino, Jackson, Taylor and Johnson2018), early Miocene deposits, especially Aquitanian, are poorly represented. In addition, studies focused on bryozoans associated with coral reefs of this age are non-existent.
This is the second part of a comprehensive taxonomic study of the bryozoan fauna from the early Miocene deposits of the Siamaná Formation in Colombia, interpreted as shallow-water coral reefs (Flórez et al., Reference Flórez, Zapata-Ramírez and Klaus2019a, Reference Flórez, Zapata-Ramírez and Klausb). In the first contribution, we described 15 species included in the order Cyclostomatida, as well as anascan-grade and Cribrilinidae Cheilostomatida. Here, we describe 17 species of ascophoran-grade Cheilostomatida, and discuss the results of both contributions.
Geologic setting
The Siamaná Formation is part of the sedimentary infill of the Cocinetas Basin, at the foothills of the Cocinas, Jarara, and Macuira mountain ranges, in the La Guajira Peninsula, a remote area of northern Colombia (Fig. 1.1). The formation has varying thickness ranging from 430 m in the type locality (Renz, Reference Renz1960) to ~20 m in the hills of Arroyo Ekieps (Fig. 1.2). It consists of basal conglomerates overlain by sandstones and fossiliferous limestones interbedded with silty clays (Rollins, Reference Rollins1965; Teatin, Reference Teatin1991). Limestones in the upper part of the Siamaná Formation include coral reefs (Rollins, Reference Rollins1965), some of which grew bordering the SE of the Jarara paleoisland (current highlands, Fig. 1). The studied bryozoan samples were collected in the framestone of these reefs, which developed in shallow waters with low siliciclastic input and therefore low turbidity (Flórez, Reference Flórez2020). The Siamaná Formation unconformably overlaps the Eocene Macarao Formation and Jurassic metamorphic rocks (Rollins, Reference Rollins1965). Deep marine sediments of the lower Miocene Uitpa Formation unconformably overlie the Siamaná Formation at the basin margins; however, the transition between both formations can be conformable and gradational in the central part (Rollins, Reference Rollins1965).
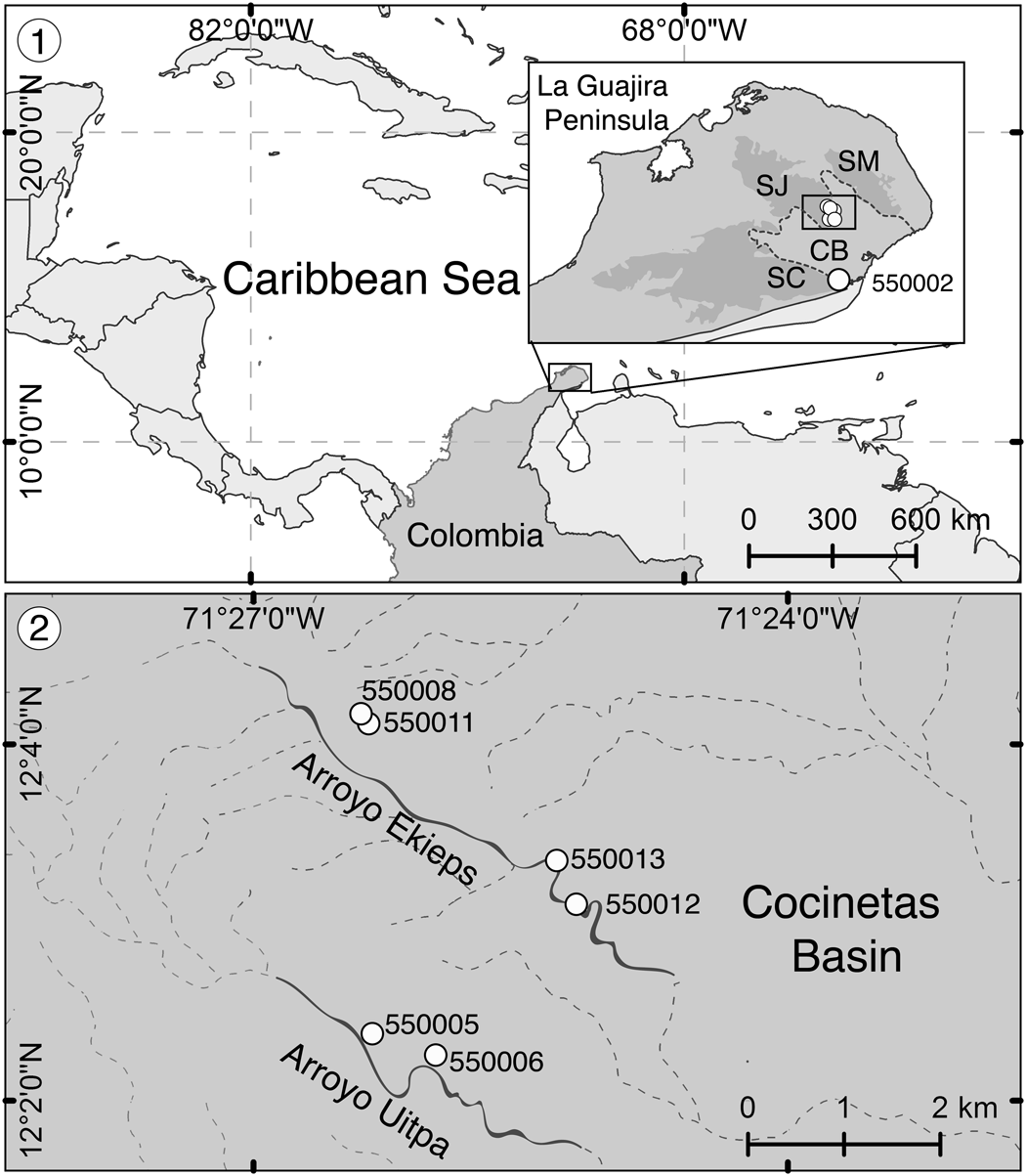
Figure 1. Locality maps from Flórez et al. (Reference Flórez, Di Martino and Ramalho2021). (1) Location of the La Guajira Peninsula in the Caribbean region and detail of the sampled zone showing the Serranía de Cocinas (SC), Serranía de Jarara (SJ), and Serranía de Macuira (SM) surrounding the Cocinetas Basin (CB) (dotted area in the box), including the locality La Flor de La Guajira (station 550002). (2) Close-up of localities Arroyo Ekieps (stations 550008, 550011, 550012, 550013) and Arroyo Uitpa (stations 550005, 550006) at the foothills of the Serranía de Jarara in the Cocinetas Basin.
The age of the Siamaná Formation continues to be a subject of debate. Some works point to a late Oligocene age (Rollins, Reference Rollins1965; Cardenas et al., Reference Cardenas, Jaramillo and Oboh-Ikuenobe2020; Jaramillo et al., Reference Jaramillo, Sepulchre, Cardenas, Correa-Metrio, Moreno, Trejos, Vallejo, Hoyos, Martínez, Carvalho, Escobar, Oboh-Ikuenobe, Prámparo and Pinzón2020), while others suggest a diachronic deposition that reached the early Miocene (Duque-Caro, Reference Duque-Caro1974; Teatin, Reference Teatin1991; Silva-Tamayo et al., Reference Silva-Tamayo, Lara, Nana Yobo, Erdal, Sanchez and Zapata-Ramírez2017). Here, we follow Flórez (Reference Flórez2020) and Flórez et al. (Reference Flórez, Di Martino and Ramalho2021) who suggested an early Miocene age based on larger benthic foraminifera, and the dating provided by Silva-Tamayo et al. (Reference Silva-Tamayo, Lara, Nana Yobo, Erdal, Sanchez and Zapata-Ramírez2017) based on strontium isotopes of coralline algae from the same localities studied here. A revision of the lateral facies change, within the early Miocene, is needed in some parts of the Cocinetas Basin to understand the stratigraphic relationships between the Siamaná and Uitpa formations and clarify the age of the last onlapping phases of the former; however, this is beyond the scope of the present work. Additional information on the geologic and stratigraphic settings of the sampling localities is provided in Flórez et al. (Reference Flórez, Zapata-Ramírez and Klaus2019a, fig. 2).
Materials and methods
Three localities and seven stations were sampled (Fig. 1; Appendix 1). Samples were collected by hand-picking along 10 m transects. The bryozoan specimens were found encrusting coral colonies, on rubble covered by coralline algae, and scattered in the sediment adhering to these hard substrates. The coral substrates were washed and the residual sediment was sieved at 250 and 63 μm. The better-preserved bryozoan colonies were cleaned with ultrasound, and later analyzed with scanning electron microscopy (SEM), employing FEI Quanta 400 and FEI Quemscan 650F microscopes operating at low- and high-vacuum modes, respectively. The morphometric measurements (including average, observed range, standard deviation and the number of measurements) were performed with the image-processing program Image J (https://imagej.nih.gov/ij), and reported in tables. The systematic paleontology follows the interim classification compiled by Gordon (Reference Gordon2014) and the work of Winston et al. (Reference Winston, Vieira and Woollacott2014). The catalog numbers and metadata of the specimens studied are supplied in Appendix 2. More details about the methods are provided in Flórez et al. (Reference Flórez, Di Martino and Ramalho2021).
Repositories and institutional abbreviations
All type specimens, as well as any additional material described and illustrated here, are stored in the reference collection of the Mapuka Museum of the Universidad del Norte, Barranquilla-Colombia (MUN-STRI). The type specimens of species used for comparison are housed in the following institutions: Museu Nacional, Universidade Federal do Rio de Janeiro, Brazil (MNRJ); Natural History Museum, London, UK (NHMUK); U.S. National Museum of Natural History, Washington, USA (USNM); and the Museum of Comparative Zoology, Cambridge, USA (MCZ).
Systematic paleontology
Superfamily Catenicelloidea Busk, Reference Busk1852
Family Catenicellidae Busk, Reference Busk1852
Genus Catenicella de Blainville, Reference de Blainville and Levrault1830
Type species
Eucratea contei Audouin, Reference Audouin and Audouin1826, from the Mediterranean Sea, Egypt and Syria, Recent; by original designation.
Catenicella sp. indet.
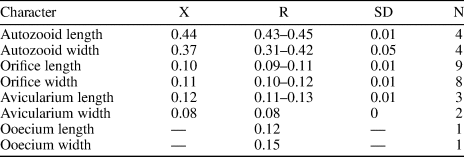
Figure 2.1–2.5; Table 1
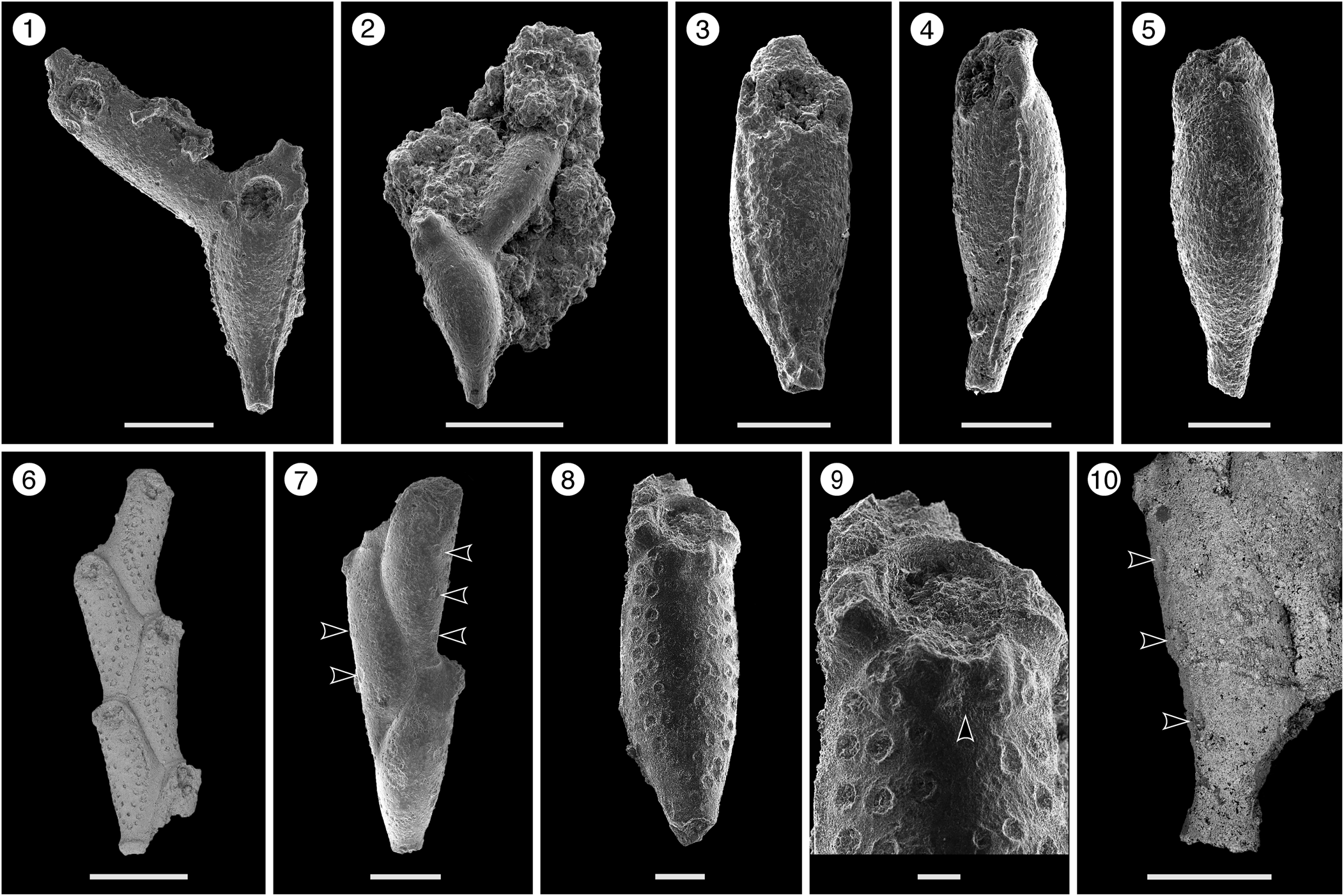
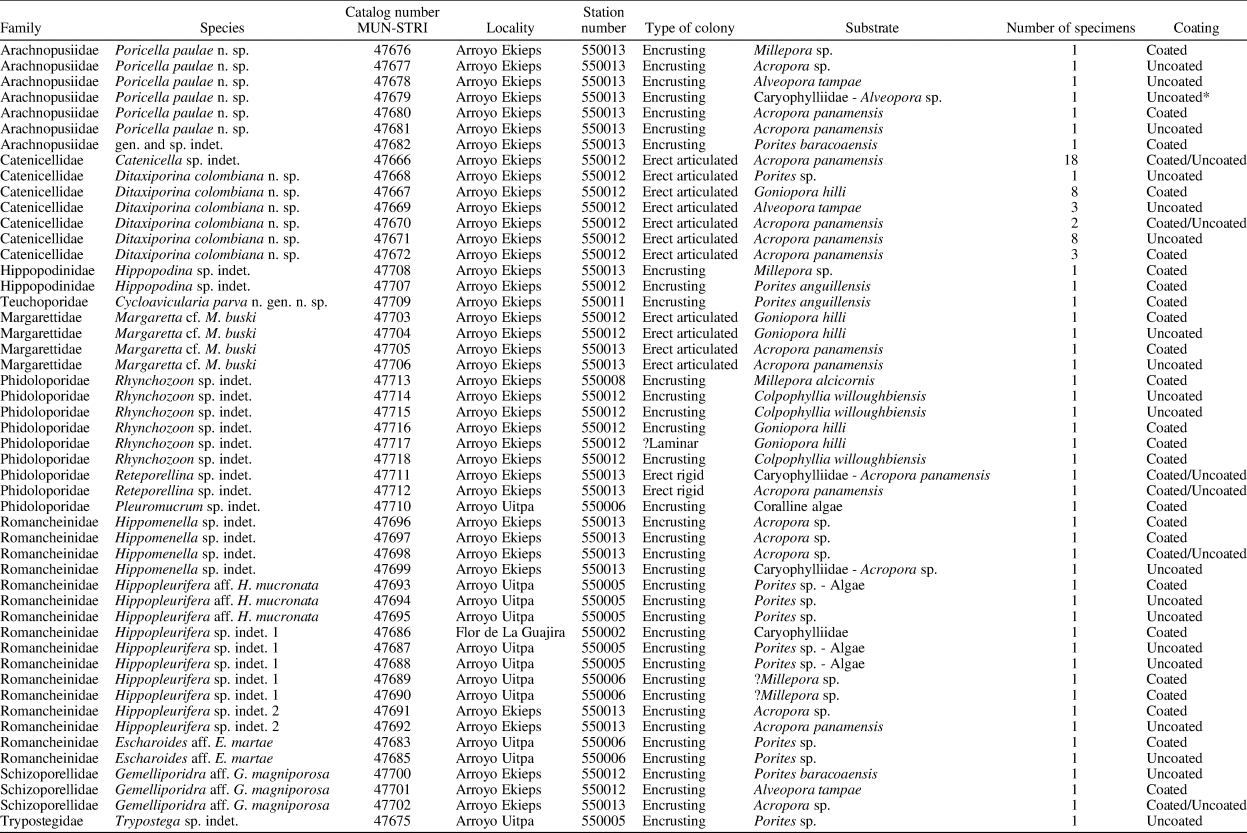
Figure 2. Catenicella sp. indet. (MUN-STRI-47666). (1) Frontal view of zooids at bifurcation, showing orifice shape, latero-oral avicularia, and location of the vittae; (2) abfrontal view of the bifurcation; (3) detail of a single zooid showing the vittae, the orifice sinus, and oral avicularia; (4) lateral view of a zooid; (5) abfrontal surface of a zooid. Ditaxiporina colombiana n. sp. (paratype MUN-STRI-47672). (6) Frontal view of a biserial internode (holotype MUN-STRI-47667); (7) abfrontal view of a biserial internode showing the lateral pores (arrowed); (8) detail of a single zooid showing the orifice shape, vestigial suboral costae, and arrangement of frontal pores; (9) detail of the orifice and the middle suture (arrowed); (10) detail of the three lateral pores (arrowed). All specimens are from the Siamaná Formation, Arroyo Ekieps locality. Scale bars are (1) 0.15 mm; (2, 7) 0.25 mm; (3–5, 8, 10) 0.1 mm; (6) 0.5 mm; (9) 0.04 mm.
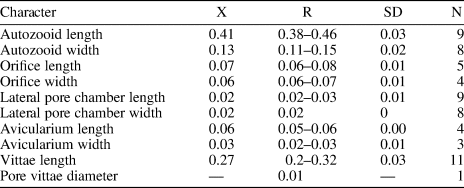
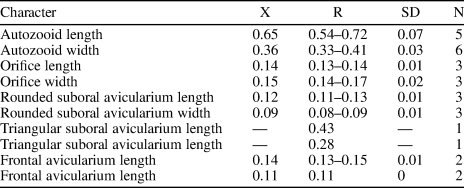
Table 1. Measurements (in mm) of Catenicella sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Occurrence
Early Miocene, Arroyo Ekieps, Siamaná Formation, La Guajira, Colombia.
Description
Colony erect, jointed, branched, and flexible. Zooids elongate (mean L/W 3.21), pyriform, uniserially arranged, all facing the same side. Gymnocyst smooth and finely perforated. Orifice subterminal, semicircular, with a slightly raised proximal lip, forming a shallow sinus and seemingly flanked by two condyles. Infrascapular chamber with a circular to elliptical pore oriented frontally. Avicularia small with triangular rostrum, placed at the sides of the orifice, oriented frontally to laterofrontally. Suprascapular chamber ?drop-shaped, oriented distally. Vittae long and narrow, placed on both sides of the zooid, bearing 12–13 circular communication pores, beginning next to the joint and ending at the base of the lateral chamber. Abfrontal surface convex and smooth. Rhizooids and ovicells not observed. Giant avicularia unknown in our specimens.
Other material compared
Catenicella uberrima (Harmer, Reference Harmer1957) Recent, Arraial do Cabo (Forno Beach), Rio de Janeiro State, Brazil, 1.5 m depth, MNRJ-136.
Remarks
Material from the Siamaná Formation resembles the modern species Catenicella uberrima described from Indonesia, and reported from western Africa (Cook, Reference Cook1968) and the western Atlantic from Florida, the Gulf of Mexico, the Caribbean, and Brazil (Winston, Reference Winston1982; Ramalho et al., Reference Ramalho, Taylor and Muricy2014; Delgadillo-G. and Flórez, Reference Delgadillo-G. and Flórez2015). Both species have elongate zooids and long vittae. However, C. uberrima is slightly larger, the pores of the infrascapular chambers and avicularia are placed laterally in regular zooids, and laterofrontally only in ovicellate zooids. In addition, at bifurcations, the non-articulated budded zooid is fused to the parental zooid for almost half of its length (e.g., Ramalho et al., Reference Ramalho, Taylor and Muricy2014, fig. 2b), while in Catenicella sp. indet. the budded zooid is fused just at the base (Fig. 2.1). Vittaticella sp. recorded by Cheetham et al. (Reference Cheetham, Jackson, Sanner and Ventocilla1999) (=Catenicella, illustrated in NMiTA Database, 1996–2016) in the Caribbean (ca. 5.9–15.7 Ma) differs from Catenicella sp. indet. in having the pore of the infrascapular chamber narrow and lanceolate, oriented proximomedially, as well as two small drop-shaped suprascapular pores, oriented almost frontally. The scarcity of material in the Siamaná Formation prevents classification at species level or the description of a new species. Catenicella sp. indet. was found in the sediment adhering to the coral Acropora panamensis (Vaughan, Reference Vaughan1919), co-occurring with the bryozoans Licornia sp., Ditaxiporina colombiana n. sp., and Reteporellina sp. This is the oldest record of the genus Catenicella in the American continent (ca. 23–20 Ma), and the first one in coral reefs ecosystems.
Genus Ditaxiporina Stach, Reference Stach1935
Type species
Catenicella septentrionalis Waters, Reference Waters1891, from Montecchio Maggiore, Italy, Priabonian (Eocene); by original designation.
Ditaxiporina colombiana new species
Figure 2.6–2.10; Table 2
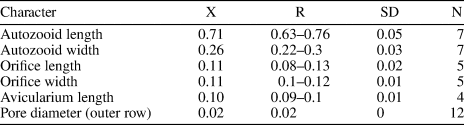
Table 2. Measurements (in mm) of Ditaxiporina colombiana n. sp. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Holotype
MUN-STRI-47667. Paratypes: MUN-STRI-47668; MUN-STRI-47669, MUN-STRI-47670, MUN-STRI-47671, MUN-STRI-47672. From the lower Miocene Siamaná Formation, Arroyo Ekieps, La Guajira, Colombia.
Diagnosis
Colony erect, branching, uni- or biserial. Biserial internodes can be without fertile zooids. Zooids claviform. Orifice semicircular, proximal border formed by two vestigial costae. Frontal shield made of smooth gymnocyst, regularly perforated by circular pores arranged in six longitudinal rows. ?Three elliptical pores placed along each side of the zooid. Abfrontal surface smooth, lacking pores. Oral avicularia single or paired, with triangular rostrum oriented distomedially and complete crossbar. Ovicell unknown.
Description
Colony erect, articulated, internodes uni- or biserial. Zooids elongate and claviform (mean L/W 2.76); biserial internodes with up to five zooids, alternated with and separated by narrow grooves, forming a zig-zag line along the center of the internode, all zooids facing the same side. Orifice terminal, semicircular with a broad sinus; proximal rim formed by two short and raised vestigial costae, ?with pelmata and separated by a suture along the middle line. Gymnocyst smooth, convex, perforated by circular and conspicuous pores with a slightly raised rim. Pores aligned forming three curved and concentric ‘Vs’; the outer series bearing 23–27 pores; the middle series, slightly smaller, having 17–19 pores; and the inner series, the smallest, with seven pores; the central area is generally imperforate, but a small, isolated pore may occur. Three elliptical to oval lateral pores along the outer lateral side of the zooid. Abfrontal surface smooth, imperforate, and convex. Avicularia small, single or paired, placed at the sides of the orifice; rostrum triangular, short, oriented distomedially; crossbar complete. Ovicell unknown. Oral spines absent.
Etymology
Named after Colombia, in reference to the country where it was collected for the first time, plus the Latin suffix -ianus, belonging to.
Remarks
The analyzed material shares the characteristics of two close genera of the subfamily Ditaxiporinae Stach, Reference Stach1935: the fossil genus Ditaxiporina, and the recent genus Vasignyella Gordon, Reference Gordon1989, the latter genus transferred to the subfamily Vasignyellinae Gordon and Braga, Reference Gordon and Braga1994 (Vieira et al., Reference Vieira, Gordon and Correia2007). Both genera have species with unizooidal and/or multizooidal internodes. In the members of Vasignyella, the multizooidal internodes are infrequent and bear ovicellate zooids, the paired avicularia lack a crossbar and bear lateral pore chambers (Vieira et al., Reference Vieira, Gordon and Correia2007, p. 51, 56). By contrast, the members of Ditaxiporina have multiserial internodes with or without fertile zooids, pelmata in the suboral costae, and single or paired avicularia with a complete crossbar (Gordon and Braga, Reference Gordon and Braga1994). Despite the scarcity of material and its poor preservation, it is possible to infer that the specimens belong to Ditaxiporina owing to the absence of ovicells or scars thereof in the multizooidal internodes. Two North American congeners are known from the early Oligocene, Ditaxiporina subseptentrionalis (Canu and Bassler, Reference Canu and Bassler1917), and Ditaxiporina bifenestrata Cheetham, Reference Cheetham1962a. The former species differs from Ditaxiporina colombiana n. sp. in having tubular frontal pores and in the absence of the suboral vestigial costae, while the latter species differs in having the orifice proportionately much smaller and a single smaller avicularium without crossbar (Cheetham, Reference Cheetham1962a). The closest congener is D. septentrionalis (Waters, Reference Waters1891), known from the Eocene of Italy (Gordon and Braga, Reference Gordon and Braga1994, fig. 10 a–d), which is similar also in the size of the autozooids; however, D. colombiana n. sp. differs in having pores in the lateral wall and three longitudinal series of frontal pores, and in the absence of a communication pore in the abfrontal surface, below the avicularia. The discovery of Ditaxiporina in the Colombian early Miocene represents the globally youngest record of the genus. In the Siamaná Formation, Ditaxiporina colombiana n. sp. was found in sediment adhering to the corals Acropora panamensis, Alveopora tampae Weisbord, Reference Weisbord1973, and Goniopora hilli Vaughan, Reference Vaughan1919, co-occurring with the bryozoans Licornia sp., Catenicella sp., Reteporellina sp., Margaretta cf. M. buski Harmer, Reference Harmer1957, Mecynoecia sp., and Poricellaria sp.
Superfamily Hippothooidea Busk, Reference Busk1859
Family Trypostegidae Gordon, Tilbrook, and Winston in Winston, Reference Winston2005
Genus Trypostega Levinsen, Reference Levinsen1909
Type species
Lepralia venusta Norman, Reference Norman1864, from English Channel, Guernsey Island, Recent; by original designation.
Trypostega sp. indet.
Figure 3; Table 3
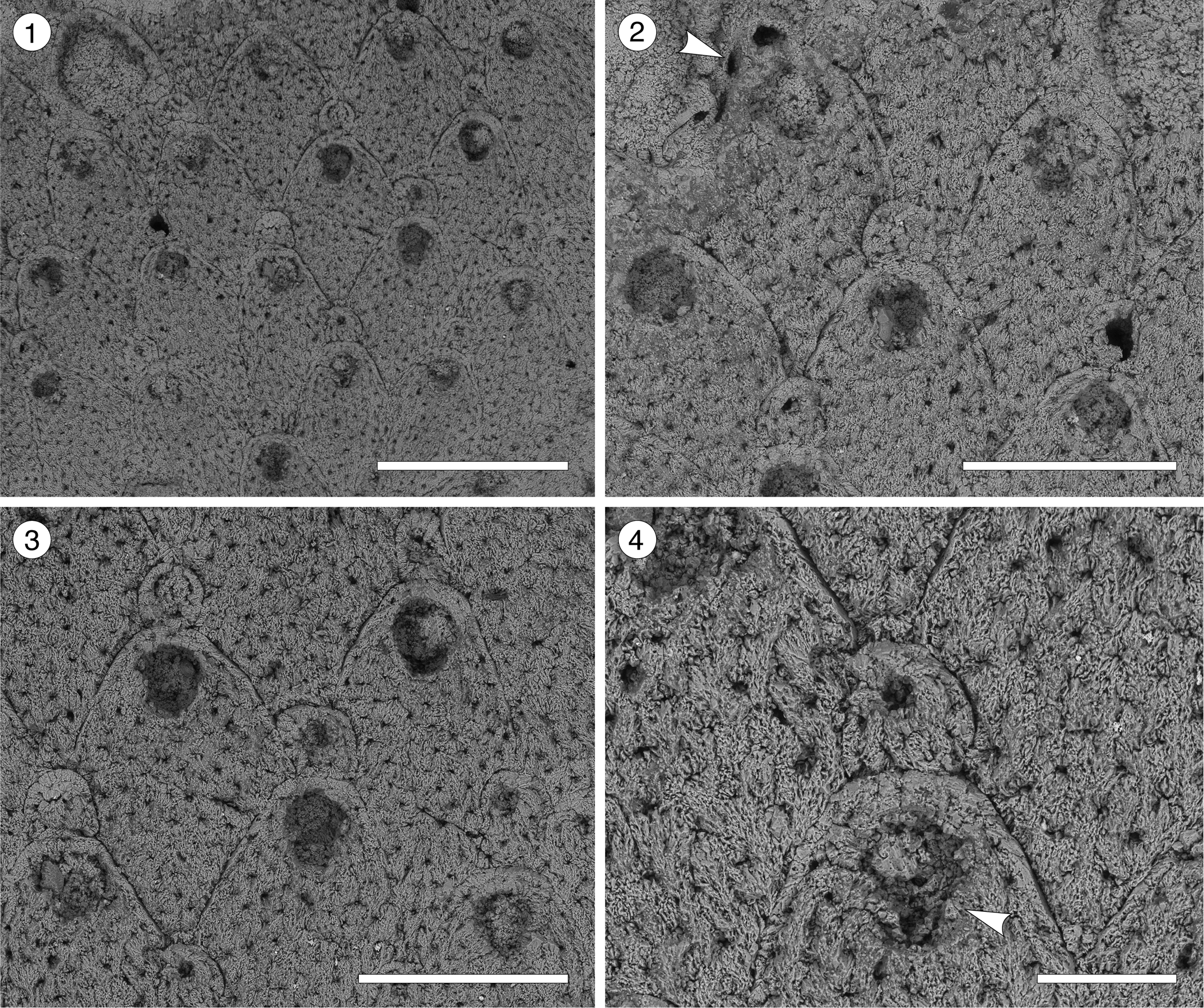
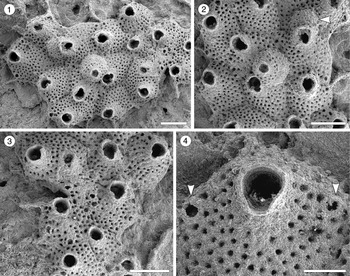
Figure 3. Trypostega sp. indet. (MUN-STRI-47675) from the Siamaná Formation, Arroyo Uitpa. (1) General view of the zooids; (2) detail of the basal pore-chambers (arrowed); (3) detail of the zooids and zooeciules; (4) detail of the orifice, sinus, and likely condyles (arrowed). Scale bars are (1) 0.5 mm; (2, 3) 0.3 mm; (4) 0.1 mm.
Table 3. Measurements (in mm) of Trypostega sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Occurrence
Early Miocene, Siamaná Formation, Arroyo Uitpa, Colombia.
Description
Colony encrusting, multiserial, unilaminar. Autozooids distinctly separated by narrow and shallow grooves, rhomboidal, longer than wide (mean L/W 1.64), arranged quincuncially. Frontal shield flat, evenly perforated by 46–58 circular pseudopores (diameter 0.01 mm). Orifice subterminal, pyriform to cleithridiate, longer than wide; anter semicircular, condyles seemingly robust and rounded, sinus U-shaped. Basal pore-chamber windows elliptical to circular. Zooeciules placed distally to almost each autozooid, subcircular to oval, similar in size to the primary opening of autozooids; opening small, ?circular, placed in the center or slightly displaced distally; frontal shield flat, evenly covered by circular pseudopores as in autozooids. Suboral umbo absent. Ovicells not observed.
Remarks
Despite the high level of recrystallization in the single specimen available, the key features of the genus Trypostega, such as the presence of zooeciules, cleithridiate orifice, and the pseudoporous pattern of the frontal shield, are clearly distinguishable. Five fossil species of this genus are known from North America: T. inornata (Gabb and Horn, Reference Gabb and Horn1862), T. elongata Canu and Bassler, Reference Canu and Bassler1920, and T. undulata (Canu and Bassler, Reference Canu and Bassler1920) from the Eocene; T. vokesi Di Martino, Taylor, and Portell, Reference Di Martino, Taylor and Portell2017, from the Miocene (Burdigalian); and T. composita Di Martino, Taylor, and Portell, Reference Di Martino, Taylor and Portell2019, from the Pliocene (Piacenzian). Trypostega inornata and T. elongata both resemble T. sp. indet. in having zooeciules associated to each autozooid, but the former species differs in having zooids with an imperforate frontal shield, while the latter species differs in having elongate and fusiform zooeciules. Trypostega composita differs in having zooeciules of variable size and shape, often forming clusters, in addition to frontal subcolonies. The remaining species differ in having a suboral umbo that is constantly present or at least developed in some areas of the colony (e.g., T. vokesi). Among Recent West Atlantic species, T. striatula (Smitt, Reference Smitt1873), T. ilhabelae Winston and Vieira, Reference Winston and Vieira2013, and T. tropicalis Winston, Vieira, and Woollacott, Reference Winston, Vieira and Woollacott2014, all differ in having a suboral umbo, and T. striatula also has longitudinal, conspicuous striations. The absence of ovicells and scarcity of specimens prevent a new species introduction. In the Siamaná Formation Trypostega sp. indet. was found growing on Porites sp., co-occurring with an indeterminate cheilostome.
Superfamily Arachnopusioidea Jullien, Reference Jullien1888
Family Arachnopusiidae Jullien, Reference Jullien1888
Genus Poricella Canu, Reference Canu1904
Type species
Poricella maconnica Canu, Reference Canu1904, from Tunisia, Eocene; by original designation.
Poricella paulae new species
Figure 4; Table 4
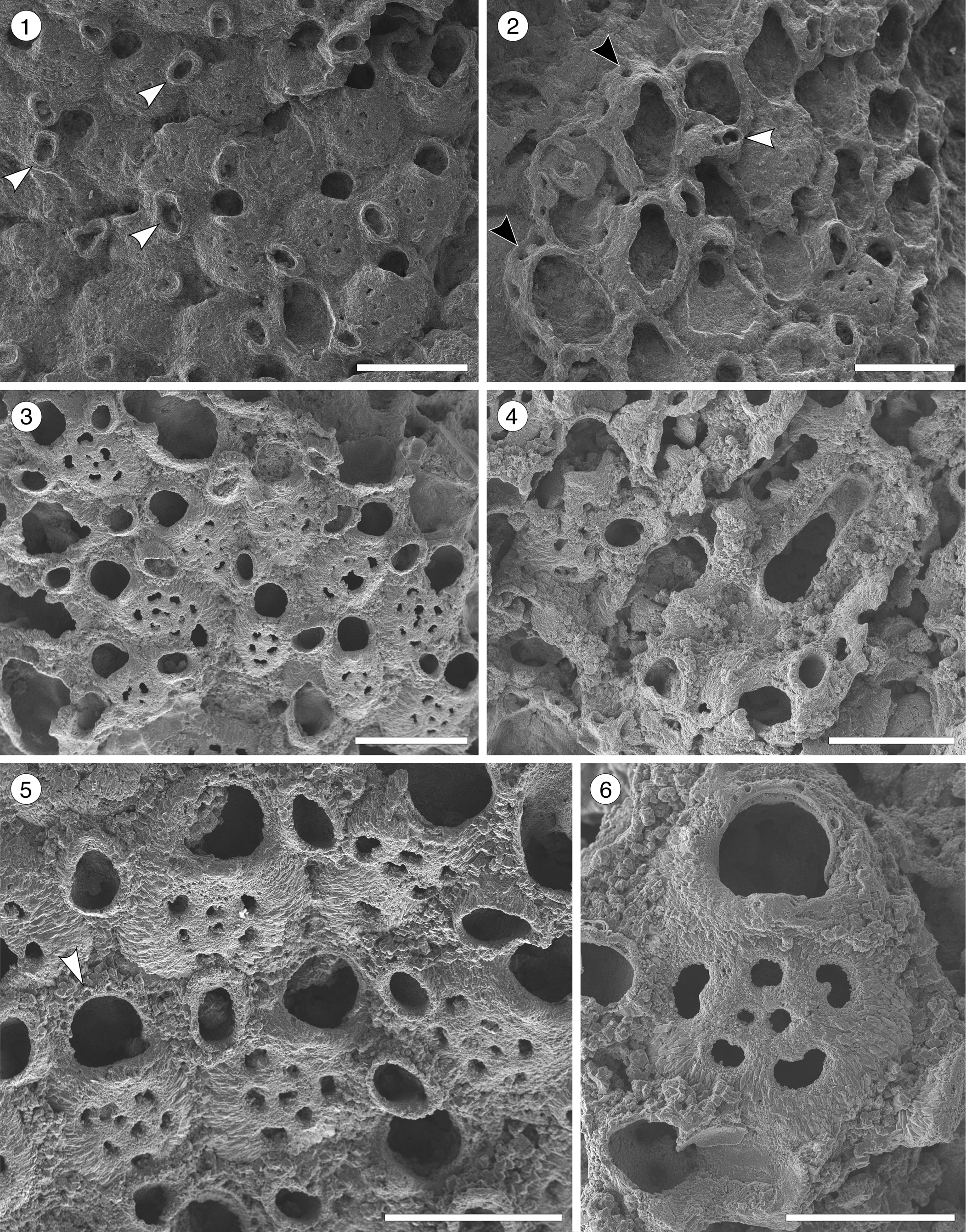
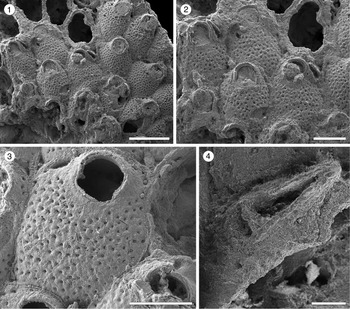
Figure 4. Poricella paulae n. sp. (paratype MUN-STRI-47680) from the Siamaná Formation, Arroyo Ekieps. (1) General view of the colony with several subspatulate avicularia (arrowed); (2) detail of the pore-chambers (black arrows) and avicularium associated with the ovicell (white arrow); (holotype MUN-STRI-47676) (3) general view of the zooids and rounded interzooidal avicularia; (4) detail of the giant elongate interzooidal avicularium; (5) detail of the orifice bearing six oral spines (arrowed); (6) detail of the frontal foramina, orifice with condyles, and interzooidal avicularium. Scale bars are (1–4) 0.5 mm; (5) 0.4 mm; (6) 0.3 mm.
Table 4. Measurements (in mm) of Poricella paulae n. sp. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Holotype
MUN-STRI-47676. Paratypes: MUN-STRI-47677, MUN-STRI-47678, MUN-STRI-47679, MUN-STRI-47680, MUN-STRI-47681. From the lower Miocene Siamaná Formation, Arroyo Ekieps, La Guajira Colombia.
Diagnosis
Colony encrusting. Autozooids distinct, ovoidal to elliptical. Orifice terminal, semicircular, slightly wider proximally; four to six oral spine bases. Frontal shield smooth, convex, perforated by 3–9 (most often 7) foramina; suboral mucro present or absent. One to three prominent, interzooidal avicularia surrounding each autozooid, usually rounded to elliptical or subspatulate. Sparse giant interzooidal avicularia, with elliptical or pyriform opesia and rounded rostrum. All avicularia lacking crossbar. Ovicell hyperstomial, globular.
Description
Colony encrusting, multiserial, multilaminar. Autozooids distinct, separated by deep furrows, ovoidal to elliptical (mean L/W 1.39), arranged in alternating rows or irregularly. Frontal shield convex with a relatively flat center, smooth, perforated by 3–9, most often seven, circular or bean-shaped foramina of different sizes; suboral mucro developed in most zooids. Marginal areolar pores few and small. Pore-chamber windows large, circular to elliptical, visible in the lateral and distal walls of marginal zooids. Orifice terminal, D-shaped; two rounded, proximally placed condyles separating a semicircular anter from a slightly wider sinus with straight to slightly concave proximal border; 4–6 oral spine bases. One to three interzooidal avicularia placed mid-lateral or distolateral to each zooid, variable in shape and size, mainly oval, sporadically subspatulate, prominent, with short rostrum oriented distally or distolaterally. Giant interzooidal avicularia less frequent, with long, straight, parallel sided, rounded rostrum and pyriform to elliptical opesia. Crossbar not observed. Ovicell hyperstomial, globular and imperforate.
Etymology
Named after researcher Paula Zapata-Ramírez (Universidad Pontificia Bolivariana), who obtained funds to undertake research on the Siamaná Formation coral reefs and collected the coral samples in 2011.
Remarks
Three fossil species and a population group of Poricella are known from southern North America and the Caribbean: P. horrida (Canu and Bassler, Reference Canu and Bassler1923) from the Miocene of Florida, P. lidgardi (Taylor and Foster, Reference Taylor and Foster1994) from the Plio-Pleistocene of Tobago, P. mucronata (Smitt, Reference Smitt1873) from the Miocene to Recent of Gulf of Mexico and Caribbean, and ‘Poricella miocenica’ (McGuirt, Reference McGuirt1941), originally described from the middle Miocene of Louisiana, and subsequently found in the middle Miocene of Florida and South Carolina (Cook, Reference Cook1977). Poricella horrida is easily distinguishable from P. paulae n. sp. in having an elongate orifice with condyles very close to the proximal border, and large interzooidal avicularia with triangular rostrum, single foramen, and conspicuous marginal areolar pores. Poricella lidgardi differs from the new species in having 1–3 foramina, adjacent zooids connected by calcified buttresses, and in the lack of condyles, oral spines, and mucro. Poricella mucronata exhibits a significant variation in the number of frontal foramina and oral spines, in the presence/absence of the suboral mucro, and in the shape and size of avicularia (Powell and Cook, Reference Powell and Cook1967; Cook, Reference Cook1977; Di Martino et al., Reference Di Martino, Taylor and Portell2017). However, some features appear to be more dominant than others, such as avicularia with truncate rostra and distal expansion, reduced number of foramina (generally three and always fewer than six), and almost equidimensional orifice. Although ‘Poricella miocenica’ is closely related to P. mucronata, Cook (Reference Cook1977, p. 131) distinguished the former species based on its similarity with Miocene species from Africa and Europe. Poricella paulae n. sp. resembles ‘P. miocenica’ sensu Cook (Reference Cook1977) in having avicularia associated with the ovicell, in the size of the orifice, and the frequency and orientation of oval/elliptical avicularia. However, it differs in the greater number of foramina (3–9 versus 1–2), and in the broader variety of interzooidal avicularia. Among Miocene European congeners, Poricella paulae n. sp. shares some features with P. areolata (Reuss, Reference Reuss1874) from Austria (on the coral Porites incrustans) and P. pouyetae (Cook, Reference Cook1977) from France. Both these species have elongate orifices, and P. pouyetae also shows conspicuous marginal areolar pores. In addition, P. areolata bears a single foramen, and despite P. pouyetae bearing seven foramina, as does P. paulae n. sp., these are located more centrally on the frontal shield. In the Siamaná Formation, Poricella paulae n. sp. was found growing on the corals Alveopora tampae, Acropora panamensis, Millepora sp., and Caryophylliidae, co-occurring with Hippopodina sp. indet., Cribrilaria multicostata Flórez, Di Martino, and Ramalho, Reference Flórez, Di Martino and Ramalho2021, Hippopleurifera sp. indet. 2, and an indeterminate cheilostome.
Arachnopusiidae gen. and sp. indet.
Figure 5; Table 5
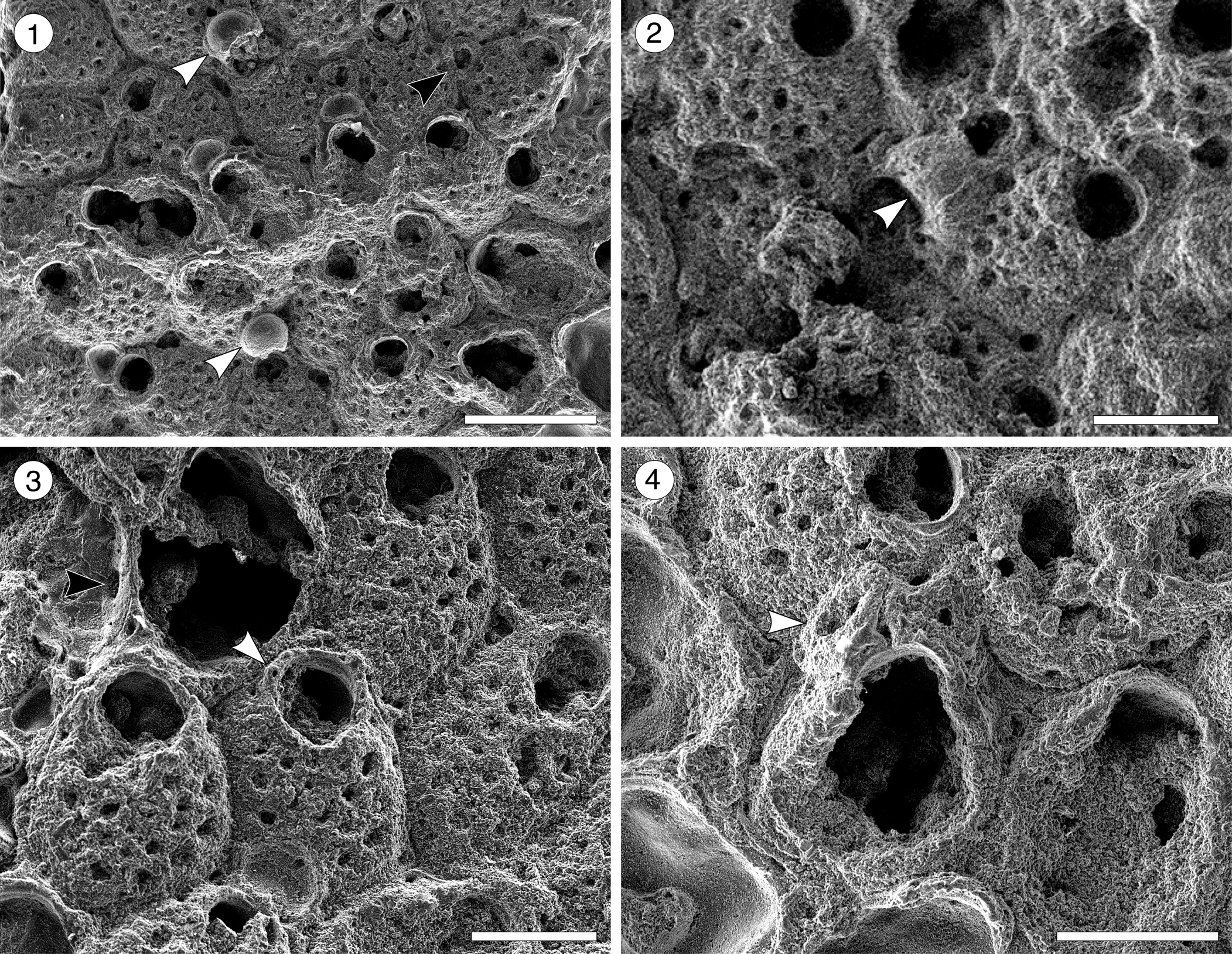
Figure 5. Arachnopusiidae gen. et sp. indet. (MUN-STRI-47682) from the Siamaná Formation, Arroyo Ekieps. (1) Fertile zooids with incomplete ectooecium exposing the smooth endooecium (white arrows), and rounded interzooidal avicularium (black arrow); (2) detail of the ovicell with the frontal window exposing the smooth endooecium (arrowed); (3) detail of the zooids showing the basal pore-chambers (black arrow) and oral spine bases (white arrow); (4) detail of the triangular adventitious avicularium (arrowed). Scale bars are 0.25 mm.
Table 5. Measurements (in mm) of Arachnopusiidae gen. et sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Occurrence
Early Miocene, Siamaná Formation, Arroyo Ekieps, Colombia.
Description
Colony encrusting, multiserial, uni- to multilaminar. Autozooids distinctly separated by deep furrows, elliptical to rounded hexagonal, longer than wide (mean L/W 1.34), irregularly arranged. Frontal shield cryptocystal, convex, perforated by 16–18 rounded to elliptical foramina (0.03 mm); marginal areolar pores subcircular to slit-like, wider at zooidal corners (0.10 × 0.05 mm); one or two large, elliptical to drop-shaped, pore-chamber windows. Orifice subcircular; a horseshoe-shaped anter separated from a slightly wider, shallow sinus by two blunt condyles; six oral spine bases in non-ovicellate zooids, four in ovicellate zooids, the proximalmost pair seemingly larger in diameter (~0.030 vs. 0.028 mm). Single, adventitious avicularium placed lateral to the orifice, triangular, oriented distally, apparently without crossbar. Interzooidal avicularia infrequent, oval, located lateral to the autozooids. Ovicells hyperstomial, subglobular; ectooecium poorly preserved, endooecium seemingly largely exposed, smooth.
Remarks
We place this specimen in the family Arachnopusiidae because of the relatively large size of the frontal surface foramina, the presence of oral spines and basal pore chambers, and the prominent ovicells (Gordon, Reference Gordon1984, p. 68). Among the genera of this family, it resembles Arachnopusia Jullien, Reference Jullien1888, in having recumbent ovicells with a frontal window exposing the endooecium; however, it differs in having autozooids with distinct outline, foramina lacking a ligula, and in the absence of suboral avicularia (Hayward and Thorpe, Reference Hayward and Thorpe1988). It also resembles Briarachnia Gordon, Reference Gordon1984, in having exposed endooecium, but Briarachnia lacks interzooidal avicularia. The poor preservation of the single specimen found in the Siamaná Formation prevents description of a new genus or species. Arachnopusiidae sp. indet. was found encrusting the coral Porites baracoaensis Vaughan, Reference Vaughan1919.
Superfamily Lepralielloidea Vigneaux, Reference Vigneaux1949
Family Romancheinidae Jullien, Reference Jullien1888
Genus Escharoides Milne-Edwards, Reference Milne-Edwards1836
Type species
Cellepora coccinea Abildgaard, Reference Abildgaard and Müller1806, from Helgoland, North Sea, Recent; by original designation.
Escharoides aff. E. martae Marcus, Reference Marcus1955
Figure 6; Table 6
- aff. Reference Marcus1955
Escharoides martae Marcus, p. 299, figs. 56, 57.
Holotype
Lost, from South of Vitória, Espírito Santo State, at 35 m depth, Brazil. Recent.
Occurrence
Early Miocene, Siamaná Formation, Arroyo Uitpa, Colombia.
Description
Colony encrusting, multiserial, unilaminar. Autozooids distinct, separated by deep grooves, oval to polygonal, slightly longer than wide (mean L/W 1.27). Frontal shield slightly convex, smooth, centrally imperforate, surrounded by a single row of circular marginal areolar pores separated by ridges. Orifice terminal, semicircular distally, hidden by the peristome proximally. Peristomial aperture shallow, two proximal sinuses formed by two robust, rounded, lateral denticles and a central ridge, bearing distally 6–7 oral spine bases (0.03 mm in diameter). Adventitious avicularia single or paired, similar in size, placed on raised, well-developed cystids outlined by a row of marginal areolar pores, located laterally adjacent to zooidal margins, at about half zooidal length; when paired, one placed more proximally than the other; rostrum triangular, oriented proximolaterally and obliquely to the frontal shield plane, crossbar complete. Ovicells not observed.
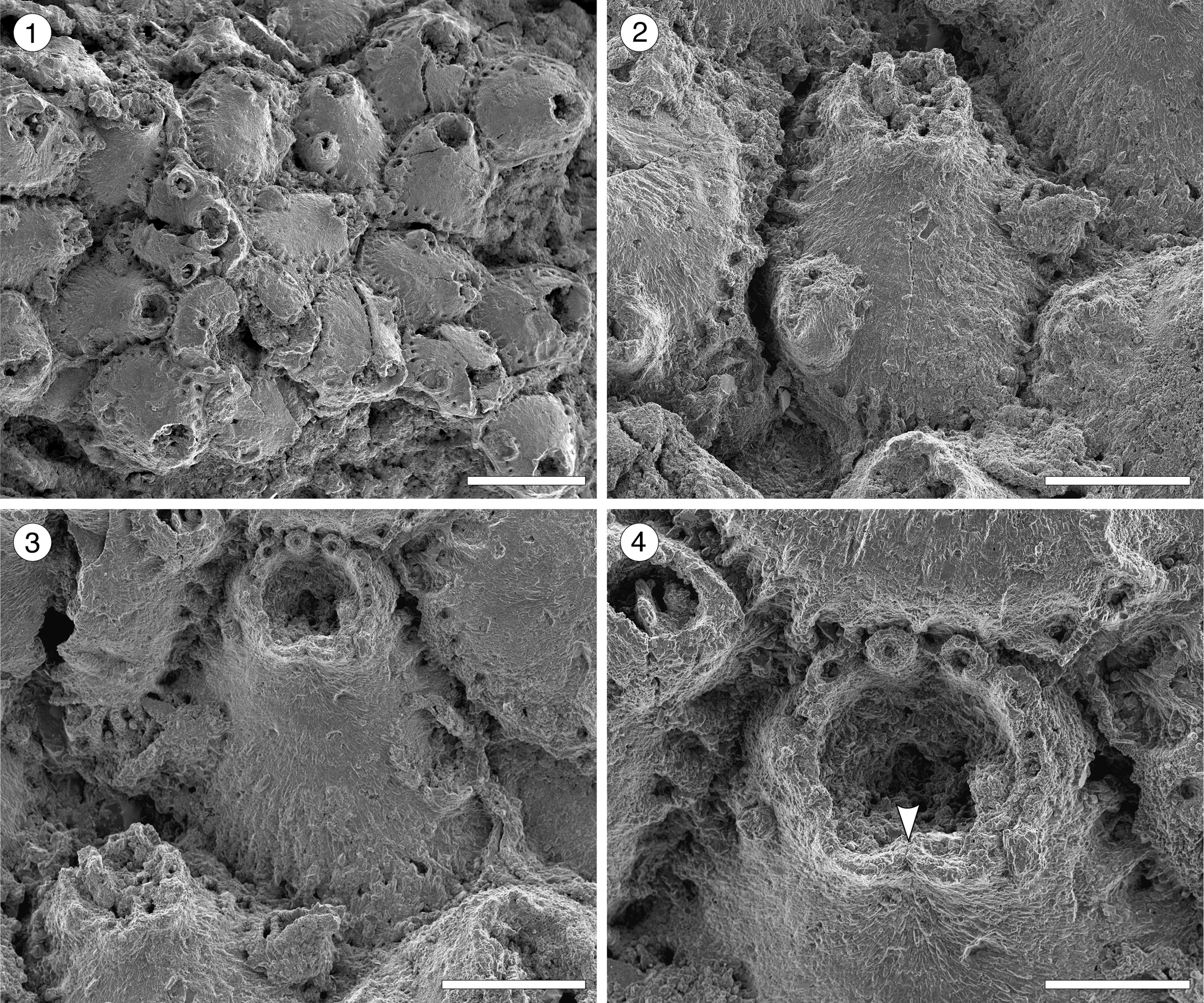
Figure 6. Escharoides aff. E. martae Marcus, Reference Marcus1955 (MUN-STRI-47683) from the Siamaná Formation, Arroyo Uitpa. (1) General view of the colony; (2) detail of a zooid and its avicularia; (3) detail of the peristomial aperture; (4) detail of the secondary orifice, central denticle (arrowed), and oral spine bases. Scale bars are (1) 0.5 mm; (2, 3) 0.2 mm; (4) 0.1 mm.
Table 6. Measurements (in mm) of Escharoides aff. E. martae. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Remarks
Canu and Bassler (Reference Canu and Bassler1920) introduced (as Peristomella) the species Escharoides falcifera and E. laticella from the Eocene, and E. erecta from the Oligocene of North America. Cheetham, Sanner, and Jackson (Reference Cheetham, Sanner and Jackson2007) described E. guraboensis from the late Miocene–early Pliocene, and Osburn (Reference Osburn1914) described E. costifer from the late Pliocene–Recent of the Caribbean region. All of these species differ from Escharoides aff. E. martae in the position and orientation of the lateral avicularia, which are placed more distally and closer to the orifice and are directed laterally, distally or distolaterally. Although these fossil specimens closely resemble the Recent E. martae from Brazil in the location, shape, and direction of the avicularia and size of autozooids, the nominal species has a more developed peristome lacking a central ridge, sparse and prominent calcified granules on the frontal shield, and larger avicularia; in addition, the mean of zooidal length/zooidal width ratio of our specimens is lower than in the Recent material (1.27 vs. 1.60). Even though E. aff. E. martae may have lost the ornament of the frontal shield by dissolution or mechanical abrasion, as seen in other Escharoides species (Berning, Reference Berning2006), the preserved morphology of the peristome distinguishes it from the nominal species. The absence of ovicells discouraged creation of a new species. In the Siamaná Formation, E. aff. E. martae was found encrusting rubble of Porites sp., sharing the substrate with Gymnophorella hadra Flórez, Di Martino, and Ramalho, Reference Flórez, Di Martino and Ramalho2021, and poorly preserved, indeterminate cribrilinids.
Genus Hippomenella Canu and Bassler, Reference Canu and Bassler1917
Type species
Lepralia mucronelliformis Waters, Reference Waters1899, from Madeira, Atlantic Ocean, Recent; by original designation.
Remarks
The definition of this genus has been puzzling since its introduction in 1917 (Tilbrook, Reference Tilbrook2006, p. 257; Berning, Reference Berning2013, p. 8; Ramalho et al., Reference Ramalho, Távora, Tilbrook and Zágoršek2015, p. 126). The absence of ovicell description in the original diagnosis of Hippomenella has led over the years to the inclusion in this genus of species with bifenestrate ectooecium, a diagnostic feature of Hippopleurifera (Berning, Reference Berning2013). In addition, discovery of fossil specimens exhibiting a combination of characters of the two genera (Di Martino and Taylor, Reference Di Martino and Taylor2015, p. 18), as in the material from the Siamaná Formation, increases the uncertainty. Here, we follow the amended diagnosis of Hippomenella in Berning (Reference Berning2013), and provide an open classification for three species: one placed in Hippomenella (based on a wider umbonuloid area, up to six oral spines, and adventitious avicularia) and two in Hippopleurifera (based on a reduced umbonuloid area, more than seven oral spines, and ovicells with bifenestrate ectooecium).
Hippomenella sp. indet.
Figure 7; Table 7
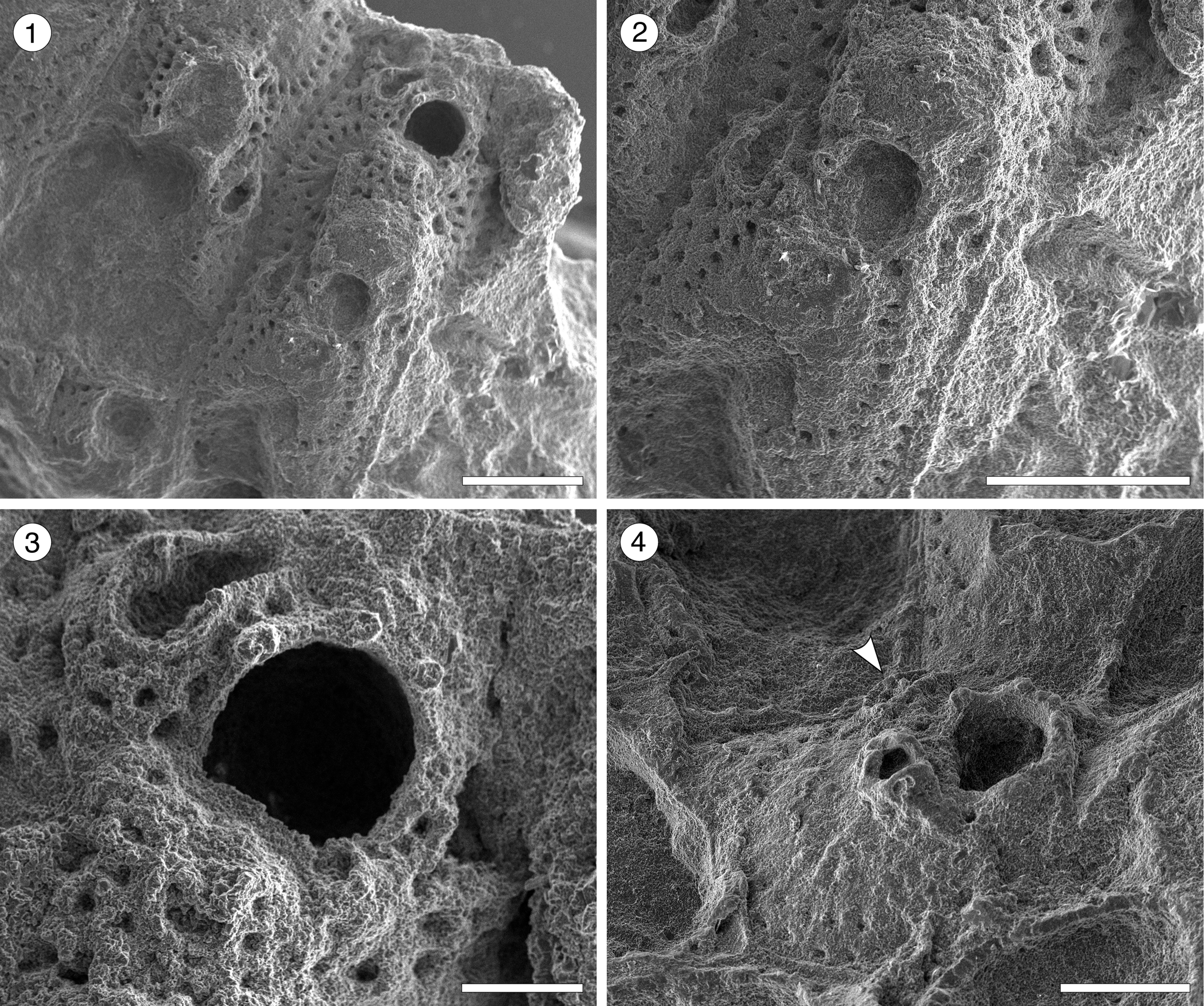
Figure 7. Hippomenella sp. indet. from the Siamaná Formation, Arroyo Ekieps. (MUN-STRI-47698) (1) general view of the zooids; (2) detail of the ooecium; (3) detail of the orifice, oral spine bases, and avicularia; (MUN-STRI-47697) (4) detail of the suboral avicularia, oral spine bases, and lateral avicularia (arrowed). Scale bars are (1, 2) 0.5 mm; (3) 0.15 mm; (4) 0.25 mm.
Table 7. Measurements (in mm) of Hippomenella sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Occurrence
Early Miocene, Siamaná Formation, Arroyo Ekieps, Colombia.
Description
Colony encrusting, multiserial, uni- to multilaminar. Autozooids separated by deep grooves, rhomboidal to claviform, longer than wide (mean L/W 1.35). Frontal shield slightly depressed marginally, raised suborally, imperforate except for 2–3 rows of elliptical, marginal areolar pores (0.03–0.04 mm in length) with radially arranged ridges in between. Orifice subcircular with straight to slightly convex proximal border; four distal oral spine bases in non-ovicellate zooids, at least two visible in ovicellate zooids. Adventitious avicularia dimorphic; a small avicularium, generally poorly preserved, placed suborally on a raised umbo; larger, distolateral avicularia placed next to the orifice, at level with the orifice proximal margin or slightly above, with triangular rostrum, oriented distolaterally and inwards, sometimes bending over the orifice. Ovicells hyperstomial, globular, surface granular.
Remarks
Our specimens consist of small colony fragments, each with only a few poorly preserved autozooids. We placed them into the genus Hippomenella based on the wide area of imperforate frontal shield, the presence of suboral and lateral avicularia, and the number of oral spines. Canu and Bassler (Reference Canu and Bassler1920) introduced the species Hippomenella transversora and ?Hippomenella pungens from the North American Oligocene; Hippomenella sp. indet. resembles both species in general appearance. However, H. tranversora has up to six oral spines, ooecium with an elongate pore, a small, triangular avicularium transversally directed, and lacks suboral avicularia, while ?H. pungens has two symmetrical avicularia placed below the level of the orifice.
Canu and Bassler (Reference Canu and Bassler1920) also described seven species of ?Hippomenella from the North American Eocene, among which Hippomenella sp. indet. resembles ?H. punctata in the suboral placement of the avicularia, but lacks the larger avicularium placed laterally to the orifice. ?Hippomenella infratelum Canu and Bassler, Reference Canu and Bassler1919, known from the Caribbean early Miocene, lacks oral spines and differs from Hippomenella sp. indet. also in having an elliptical avicularium with a complete crossbar placed more proximally on the autozooid. In the Siamaná Formation, Hippomenella sp. indet. was found on the corals Caryophylliidae and Acropora sp., co-occurring with Figularia bragai Flórez, Di Martino, and Ramalho, Reference Flórez, Di Martino and Ramalho2021, Hippopleurifera sp. indet. 2, Gemelliporidra aff. G. magniporosa, and an indeterminate cribrilinid.
Genus Hippopleurifera Canu and Bassler, Reference Canu and Bassler1925
Type species
Eschara biauriculata Reuss, Reference Reuss1847, from Eisenstadt, Mörbisch, and Kroisbach (Austria), and Oedenburg (Hungary), Miocene; by original designation.
Hippopleurifera aff. H. mucronata (Smitt, Reference Smitt1873)
Figure 8; Table 8
- aff. Reference Winston2005
Hippopleurifera mucronata; Winston, p. 54, figs. 143–145.
- aff. Reference Di Martino, Taylor and Portell2017
Hippopleurifera mucronata; Di Martino et al., p. 151, fig. 41a–d.
- aff. Reference Di Martino, Taylor and Portell2019
Hippopleurifera mucronata; Di Martino et al., p. 31, fig. 26.
Syntype
MCZ 22, Smitt collection, from Florida, at 53 m depth, USA. Recent (Winston, Reference Winston2005).
Occurrence
Early Miocene, Siamaná Formation, Arroyo Uitpa, Colombia.
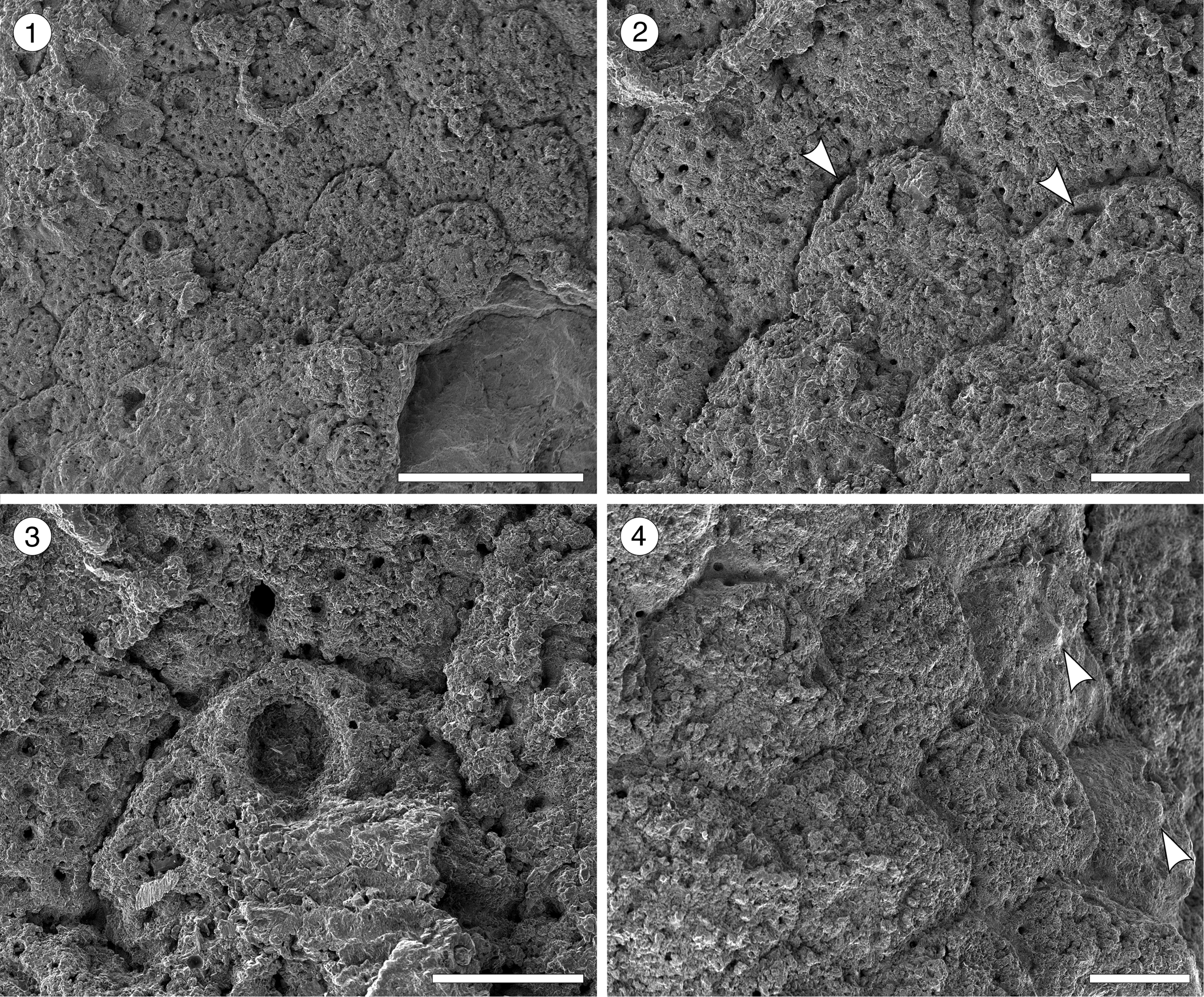
Figure 8. Hippopleurifera aff. H. mucronata (Smitt, Reference Smitt1873) from the Siamaná Formation, Arroyo Uitpa. (MUN-STRI-47693) (1) general view of the colony; (2) detail of the zooids and avicularia (arrowed); (3) detail of the orifice showing the U-shaped sinus and oral spine bases; (4) zooids showing the suboral umbo (arrowed). Scale bars are (1) 1 mm; (2, 4) 0.25 mm; (3) 0.2 mm.
Table 8. Measurements (in mm) of Hippopleurifera aff. H. mucronata. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Description
Colony encrusting, multiserial, unilaminar. Autozooids distinctly separated by narrow and deep grooves, rhomboidal, claviform, or hexagonal, almost as long as wide (mean L/W 1.05). Frontal shield convex, ribbed, with 2–3 rows of marginal areolar pores, evenly spaced, subcircular (0.02–0.03 mm in diameter), imperforate central area reduced. Orifice with semielliptical anter and narrow, U-shaped sinus; condyles not preserved; pointed suboral umbo poorly preserved; six distolateral oral spine bases. Adventitious avicularia infrequent, placed at about the same level of the oral sinus, near and parallel to zooidal margins; rostrum triangular, narrow, oriented proximolaterally; crossbar not preserved. Ovicells not observed.
Remarks
The poorly preserved specimen available resembles the nominal species Hippopleurifera mucronata in shape of the orifice, number of oral spines, multiple rows of marginal areolar pores, and position and shape of the avicularia. Compared to other records of H. mucronata, the Colombian material differs in the position and orientation of avicularia (e.g., in Di Martino et al., Reference Di Martino, Taylor and Portell2017, fig. 41a, d, the avicularium is directed almost transversally), and in the extension of the imperforate area of frontal shield (e.g., in both Winston, Reference Winston2005, fig. 143, and Di Martino et al., Reference Di Martino, Taylor and Portell2017, the imperforate area is larger). This latter character also distinguishes H. aff. H. mucronata from the North American Oligocene H. capitimortis Canu and Bassler, Reference Canu and Bassler1920, and H. ampla Canu and Bassler, Reference Canu and Bassler1920. In the Siamaná Formation, H. aff. H. mucronata was found encrusting rubble of the coral Porites sp. and coralline algae, co-occurring with Smittipora sp. indet. (Flórez et al., Reference Flórez, Di Martino and Ramalho2021) and Trypostega sp. indet.
Hippopleurifera sp. indet. 1
Figure 9; Table 9
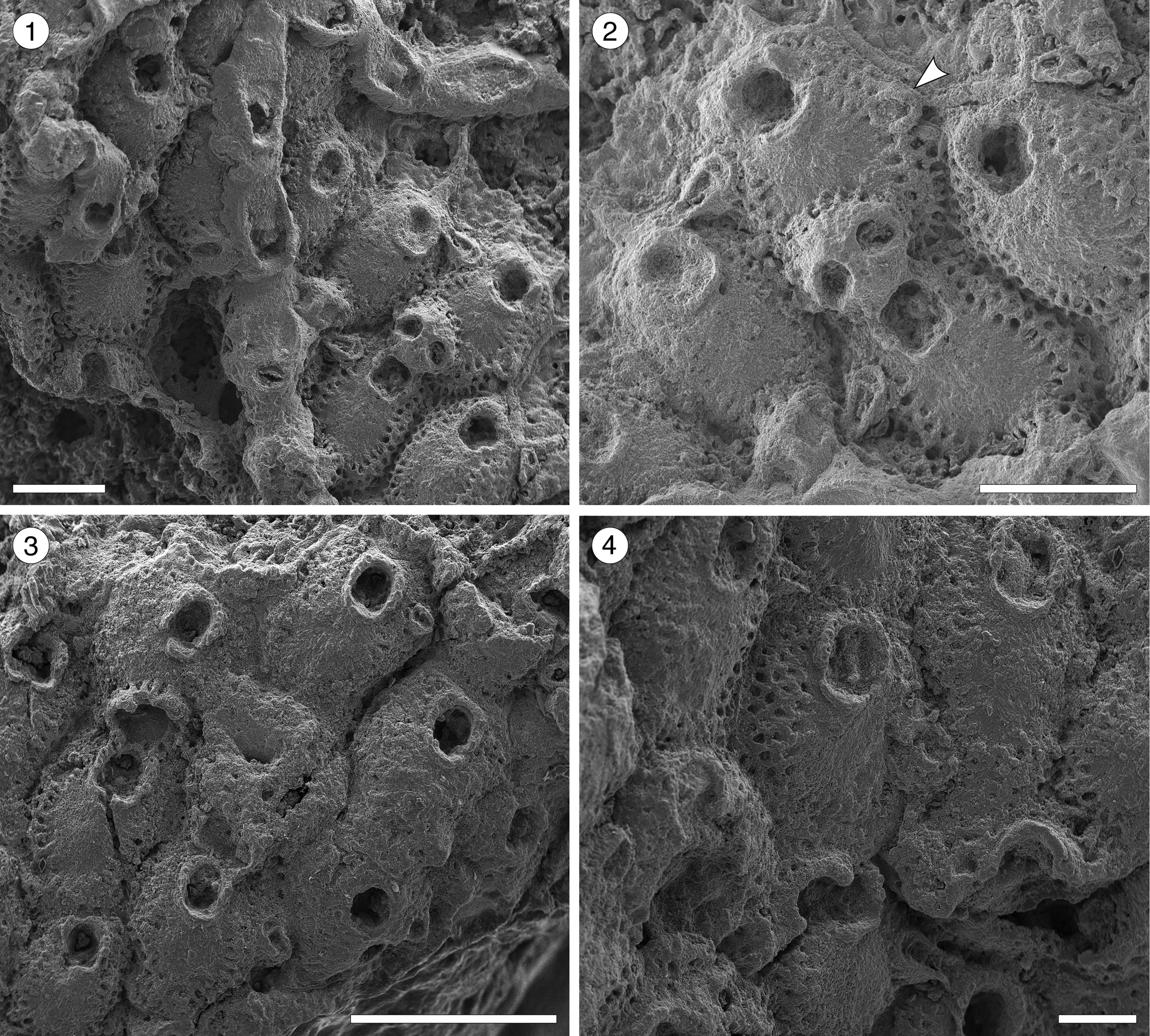
Figure 9. Hippopleurifera sp. indet. 1 from the Siamaná Formation, Arroyo Uitpa. (MUN-STRI-47690) (1) general view of the colony; (2) detail of the fertile zooid, showing the ridged and bifenestrate ectooecium and the elliptical avicularium (arrowed); (MUN-STRI-47689) (3) group of autozooids; (4) detail of the orifice and suboral peristome, oral spines, and marginal pores. Scale bars are (1, 2) 0.5 mm; (3) 1 mm; (4) 0.25 mm.
Table 9. Measurements (in mm) of Hippopleurifera sp. indet. 1. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Occurrence
Early Miocene, Siamaná Formation, Arroyo Uitpa and Flor de La Guajira, Colombia.
Description
Colony encrusting, multiserial, unilaminar. Autozooids distinctly separated by deep grooves, elliptical to subhexagonal, slightly longer than wide (mean L/W 1.22). Frontal shield slightly convex, central U-shaped area imperforate, flanked by 3–4 rows of circular, elliptical, or drop-shaped areolar pores (0.04 mm long × 0.03 mm wide) sloping inwards and separated by ridges. Orifice slightly longer than wide with arched anter separated from a smaller concave poster by two blunt condyles; 8–10 distolateral oral spine bases in non-ovicellate zooids, four in ovicellate zooids; suboral peristome well developed. Adventitious avicularia present or absent, one or two; one placed on the lateral margin of the zooid among the rows of areolar pores at about the same level of the orifice condyles, with triangular rostrum oriented laterally to proximolaterally, crossbar complete; in about half of the zooids, a second, smaller, oval to elliptical avicularium, apparently without crossbar, was observed, also placed over the rows of areolar pores, but generally on the opposite side of the zooid with respect to the oral avicularium, and more proximally. Ovicell hyperstomial, globular, slightly flattened centrally, surrounded by a row of marginal pores with radial ridges in between; ectooecium surface with radial ribs and two large drop-shaped fenestrae (0.04 mm long × 0.03 mm wide).
Remarks
Although the specimens studied here share some features with the type species of Hippomenella (see Berning, Reference Berning2013) (e.g., the wide umbonuloid area and the presence of dimorphic adventitious avicularia), we assigned them to Hippopleurifera based on the characters of the ectooecium, which is bifenestrate, and the presence of 10 or more oral spines (Tilbrook, Reference Tilbrook2006; Ramalho et al., Reference Ramalho, Távora, Tilbrook and Zágoršek2015). Twelve species of Hippopleurifera are known from the American continent, ten are fossil and two are Recent. Cheetham (Reference Cheetham1962b) introduced H. mcbeanensis, and Canu and Bassler (Reference Canu and Bassler1920) described H. incondita, H. radicata, and H. rotula, all species from the Eocene of the southeastern USA; the latter authors also described H. costulata, H. crassicollis, H. ampla, and H. capitimortis from the Oligocene–Miocene of Alabama, USA; Ramalho et al. (Reference Ramalho, Távora, Tilbrook and Zágoršek2015) introduced H. confusa and H. barbosae from the Miocene of Brazil; H. mucronata (Smitt, Reference Smitt1873) and H. belizae Winston, Reference Winston1984, are known from the Recent fauna of the Gulf of Mexico and the Caribbean.
Among the Oligocene–Miocene to Recent species, Hippopleurifera sp. indet. 1 resembles H. costulata in having a bifenestrate ooecium with radial ribs, and in the shape, size, and orientation of the oral avicularium; however, the latter species differs in having a single row of areolar pores, 4–6 oral spines, and in the absence of dimorphic avicularia. The bifenestrate ooecium and arrangement of the areolar pores also resemble H. capitimortis; however, in this species the ectooecium lacks the ribs, the fenestrae bear a proximal tongue, and the avicularia are absent or smaller in size and placed far from the orifice. In the Siamaná Formation, Hippopleurifera sp. indet. 1 was found encrusting the hydrocoral Millepora sp. and Caryophylliidae, as well as coralline algae covering the coral Porites sp., co-occurring with Gymnophorella hadra and Antropora guajirensis Flórez, Di Martino, and Ramalho, Reference Flórez, Di Martino and Ramalho2021.
Hippopleurifera sp. indet. 2
Figure 10; Table 10
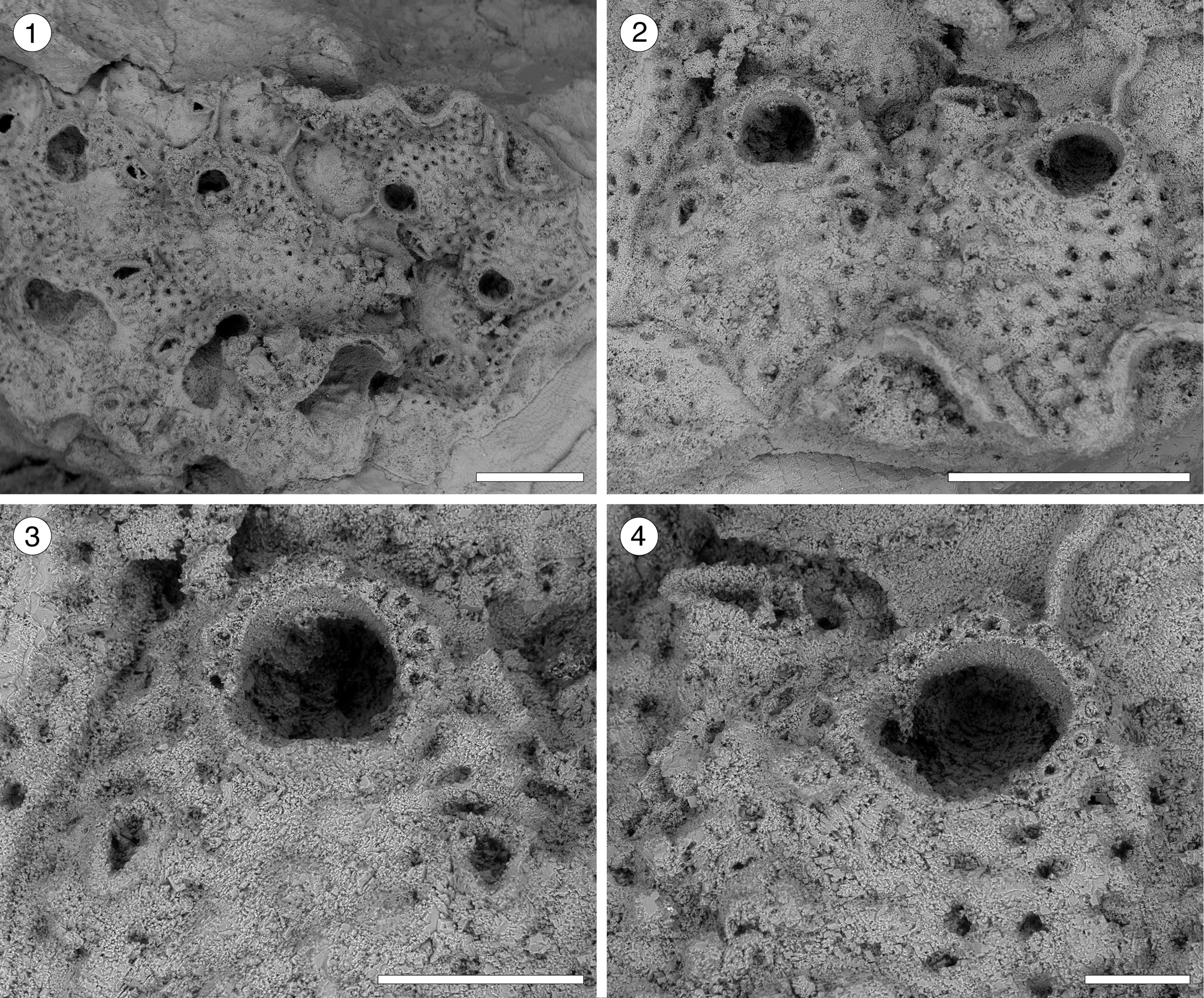
Figure 10. Hippopleurifera sp. indet. 2 (MUN-STRI-47691) from the Siamaná Formation, Arroyo Ekieps. (1) General view of the colony; (2) detail of two zooids showing the arrangement of the areolar pores, frontal avicularia, and lateral avicularium; (3) detail of the orifice, oral spine bases and paired frontal avicularia; (4) detail of the orifice, showing a condyle and oral spine bases, and a lateral avicularium. Scale bars are (1, 2) 0.5 mm; (3) 0.2 mm; (4) 0.1 mm.
Table 10. Measurements (in mm) of Hippopleurifera sp. indet. 2. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Occurrence
Early Miocene, Siamaná Formation, Arroyo Ekieps, Colombia.
Description
Colony encrusting, multiserial, unilaminar. Autozooids distinct by shallow interzooidal grooves, subhexagonal, rounded distally, slightly longer than wide (mean L/W 1.27). Frontal shield almost flat to slightly depressed, granular, central area imperforate, 3–4 rows of areolar pores. Orifice longer than wide, arched anter separated from the smaller and narrower poster (proximal border straight to slightly concave) by two blunt, rounded condyles; nine distolateral oral spine bases. One or two small, adventitious avicularia with raised, acutely triangular rostrum, oriented proximally to proximolaterally; when paired, avicularia placed symmetrically close to the lateral zooidal margins, almost at zooidal mid-length; occasionally a similar, slightly larger adventitious avicularium located laterally at the same level of the orifice, oriented lateroproximally; crossbar complete. Ovicells not observed.
Remarks
The absence of ovicells in our specimens increases the uncertainty of its classification. However, we place this species in the genus Hippopleurifera based on the relatively reduced imperforate frontal area, the high number of oral spines, and the absence of dimorphic avicularia (Tilbrook, Reference Tilbrook2006; Ramalho et al., Reference Ramalho, Távora, Tilbrook and Zágoršek2015). Among the eight species known from the American continent (see Remarks of Hippopleurifera sp. indet. 1), Hippopleurifera sp. indet. 2 resembles the Recent Caribbean species Hippopleurifera belizae Winston, Reference Winston1984, in the shape and location of the avicularia, as well as in its general aspect, but differs in the number of oral spines (9 instead of 6–8), and the morphology of the orifice, which is hoof-shaped instead of D-shaped. In the Siamaná Formation, Hippopleurifera sp. indet. 2 was found encrusting the coral Acropora panamensis and Acropora sp., co-occurring with Hippomenella sp. indet., Figularia bragai, Cribrilaria multicostata Flórez, Di Martino, and Ramalho, Reference Flórez, Di Martino and Ramalho2021, Cribrilaria nixor Flórez, Di Martino, and Ramalho, Reference Flórez, Di Martino and Ramalho2021, Gemelliporidra aff. G. magniporosa, Poricella paulae n. sp., and other indeterminate cribrilinids.
Superfamily Schizoporelloidea Jullien, Reference Jullien1883
Family Schizoporellidae Jullien, Reference Jullien1883
Genus Gemelliporidra Canu and Bassler, Reference Canu and Bassler1927
Type species
Gemelliporidra typica Canu and Bassler, Reference Canu and Bassler1927, from north of Cuba, Caribbean Sea, Recent; by original designation.
Gemelliporidra aff. G. magniporosa (Canu and Bassler, Reference Canu and Bassler1923)
Figure 11.1–11.3; Table 11
- aff. Reference Canu and Bassler1923
Schizoporella magniporosa Canu and Bassler, p. 95, pl. 45, figs. 1, 2.
- aff. Reference Winston1986
Gemelliporidra magniporosa; Winston, p. 19, fig. 41.
Syntype
USNM 68535, from Mount Hope (Canal Zone), Panama. Pleistocene.
Occurrence
Early Miocene, Siamaná Formation, Arroyo Ekieps, Colombia.
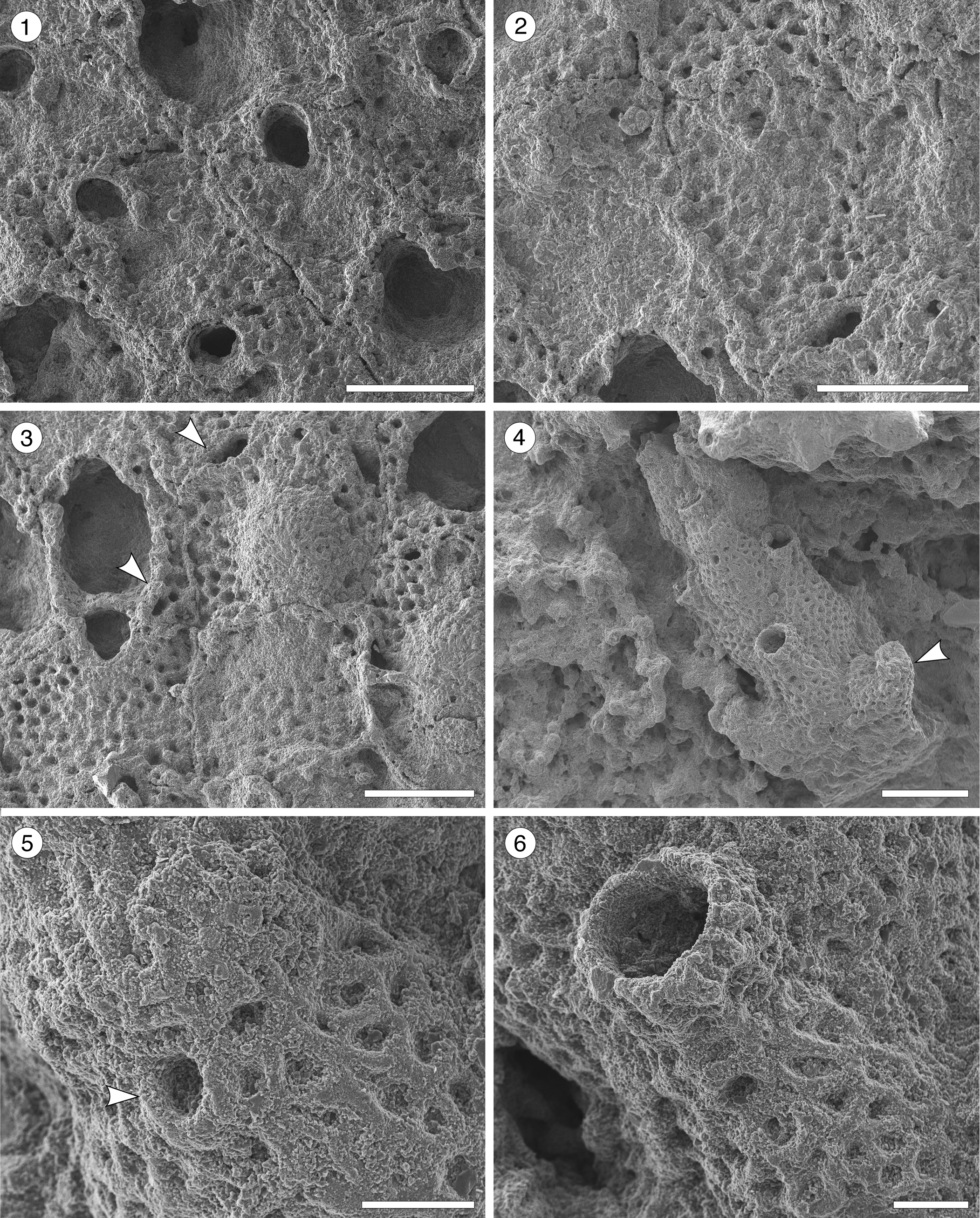
Figure 11. Gemelliporidra aff. G. magniporosa Canu and Bassler, Reference Canu and Bassler1923 (MUN-STRI-47701). (1) Detail the of zooidal shape; (2) detail of the orifice and pattern of pseudopores on the frontal shield; (3) detail of the orifice showing the V-shaped sinus, ovicell, and putative avicularia (arrowed). Margaretta cf. M. buski Harmer, Reference Harmer1957 (MUN-STRI-47705). (4) Branch fragment showing the upturned peristome of the fertile zooids (arrowed); (5) detail of the circular ascopore (arrowed); (6) detail of the peristome and secondary orifice. All illustrated specimens are from the Siamaná Formation, Arroyo Ekieps. Scale bars are (1–4) 0.5 mm; (5, 6) 0.1 mm.
Table 11. Measurements (in mm) of Gemelliporidra aff. G. magniporosa. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Description
Colony encrusting, multiserial, unilaminar. Autozooids distinctly separated by a narrow groove or a thin thread, subrectangular to irregularly pentagonal, almost as long as wide (mean L/W 1.07). Frontal shield flat to slightly depressed, granular, evenly perforated by regularly spaced, circular pseudopores (diameter 0.02–0.04 mm), except for a reduced imperforate area below the orifice. Orifice terminal, anter semielliptical, sinus rounded V-shaped, condyles rounded triangular. Small, triangular structures, seemingly oriented proximolaterally, placed at the distal zooidal margins, interpreted as putative adventitious avicularia. Ovicell hyperstomial, globular, occupying most of the frontal surface of the next distal zooid, up to the proximal margin of the orifice; apparently perforated by closely spaced pseudopores, smaller than those of the frontal shield.
Remarks
Eight of the nine species of Gemelliporidra known to date are extant and recorded off the American continent: G. colombiensis Osburn, Reference Osburn1952, and G. lata Osburn, Reference Osburn1952, from the Pacific coast of Colombia and California, respectively; G. aculeata Canu and Bassler, Reference Canu and Bassler1928, and G. pertusa (Smitt, Reference Smitt1873) from the Gulf of Mexico; G. multilamellosa Canu and Bassler, Reference Canu and Bassler1923, G. typica Canu and Bassler, Reference Canu and Bassler1927, and G. belikina Winston, Reference Winston1984, from the Caribbean Sea; and G. magniporosa Canu and Bassler, Reference Canu and Bassler1923, from both the Gulf of Mexico and the Caribbean Sea. The size, shape, and position of the putative adventitious avicularia distinguish Gemelliporidra aff. G. magniporosa from all these congeners: G. typica, G. multilamellosa, and G. aculeata have larger, straight or curved avicularia; G. belikina and G. colombiensis have small, rounded to drop-shaped avicularia placed proximolateral to the orifice; avicularia are proximolateral to the orifice in G. lata: avicularia are oval and placed on the peristome in G. pertusa.
We assigned our specimens to G. aff. G. magniporosa based on the morphology of the autozooids, orifice, and ovicells, as well as the calcification of the frontal shield and the pattern of perforation. However, G. aff. G. magniporosa differs from the nominal species in having larger autozooids (Lz 0.75 mm, Wz 0.70 mm vs. Lz 0.65 mm, Wz 0.50–0.55 mm) and orifices (Lo 0.22 mm, Wo 0.18 vs. Lo 0.17 mm, Wo 0.12), while the mean length/width ratios of both autozooids (1.07 vs. 1.18) and orifices (1.21 vs. 1.50) are lower. In addition, our specimens lack the paired, small, triangular avicularia placed at the sides of the orifice and oriented distomedially, as commonly found in G. magniporosa. In the Siamaná Formation, Gemelliporidra aff. G. magniporosa was found encrusting the corals Porites baracoaensis, Alveopora tampae, and Acropora sp., sharing the substrate with Calpensia caribensis Flórez, Di Martino, and Ramalho, Reference Flórez, Di Martino and Ramalho2021, Figularia bragai Flórez, Di Martino, and Ramalho, Reference Flórez, Di Martino and Ramalho2021, Hippomenella sp. indet., Hippopleurifera sp. indet. 2, and indeterminate cribrilinids.
Family Margarettidae Harmer, Reference Harmer1957
Genus Margaretta Gray, Reference Gray and Dieffenbach1843
Type species
Cellaria cereoides Ellis and Solander, Reference Ellis and Solander1786, from Algeria, Mediterranean Sea, Recent; by original designation.
Margaretta cf. M. buski Harmer, Reference Harmer1957
Figure 11.4–11.6; Table 12
- cf. Reference Canu and Bassler1928
Tubucellaria cereoides; Canu and Bassler, p. 113, pl. 15, fig. 6.
- cf. Reference Harmer1957
Margaretta buski Harmer, p. 834, pl. 55, fig. 29, text fig. 91.
- cf. Reference Ramalho, Serrano, Rueda, Távora and Zágoršek2019
Margaretta cf. buski; Ramalho et al., p. 112, fig. 3.
Holotype
NHMUK 87.12.9.439, from St. Paul's Rocks, shallow water, equatorial Atlantic Ocean, Brazil. Recent.
Occurrence
Early Miocene, Siamaná Formation, Arroyo Ekieps, Colombia.
Table 12. Measurements (in mm) of Margaretta cf. M. buski. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Description
Colony erect, articulated. Autozooids flask-shaped, more than twice as long as wide (mean L/W 2.35), arranged in alternating whorls of three. Frontal shield evenly perforated by large, circular pseudopores, arranged in rows between ridges; circular ascopore placed medially at the base of the peristome. Peristome tubular, relatively short, ridged, and pseudoporous as the frontal shield. Fertile zooids with peristomes swollen proximally, upturned, and tapering distally. Secondary orifice circular, primary orifice hidden by the peristome. Basis rami bipartite.
Remarks
Six fossil species of Margaretta were recorded from the American continent in addition to M. buski. Canu and Bassler (Reference Canu and Bassler1920) introduced (as Tubucellaria) the species M. fallax, M. nodifera, and M. parviporosa from the Eocene of Alabama, Florida, and North Carolina, respectively, and M. vicksburgica from the Oligocene of Alabama. Margaretta cf. M. buski differs from all these species in having smaller zooids (<1 mm long); in addition, M. nodifera has zooids with tuberosities. The Eocene species M. congesta (Cheetham, Reference Cheetham1963) and the Miocene species M. pentaceratops Di Martino, Taylor, and Portell, Reference Di Martino, Taylor and Portell2017, both from Florida, differ from M. cf. M. buski in having eight rows of smaller and densely packed zooids and peristome with five spiniform processes, respectively. Margaretta cf. M. buski resembles the Miocene to Recent M. cereoides (Ellis and Solander, Reference Ellis and Solander1786), known from the Mediterranean and East Atlantic, in general appearance, including the shape and arrangement of autozooids; however, it differs in having bipartite basis rami instead of undivided, and three zooids per whorl instead of four or five (Harmer, Reference Harmer1957). The early Miocene Siamaná specimen differs from the holotype of the nominal species (see Di Martino et al., Reference Di Martino, Taylor and Portell2017, fig. 49) in having curved but not inturned fertile peristomes, which also has been observed in specimens from the Miocene Pirabas Formation of Brazil (Ramalho et al., Reference Ramalho, Serrano, Rueda, Távora and Zágoršek2019, fig. 3). However, the limited amount of material available prevents determination of whether this is a genuine morphological difference or a diagenetic effect. The small fragments of Margaretta cf. M. buski were found in sediment cemented to the corals Goniopora hilli and Acropora panamensis, co-occurring with Nellia cf. N. tenella (Lamarck, Reference Lamarck1816), Ditaxiporina colombiana n. sp., Mecynoecia sp., Catenicella sp., Reteporellina sp., and Glabrilaria sp.
Family Hippopodinidae Levinsen, Reference Levinsen1909
Genus Hippopodina Levinsen, Reference Levinsen1909
Type species
Lepralia feegeensis Busk, Reference Busk1884, from Bisayas Sea, Philippines, Recent; by original designation.
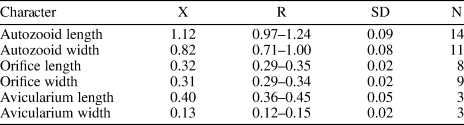
Hippopodina sp. indet.
Figure 12; Table 13
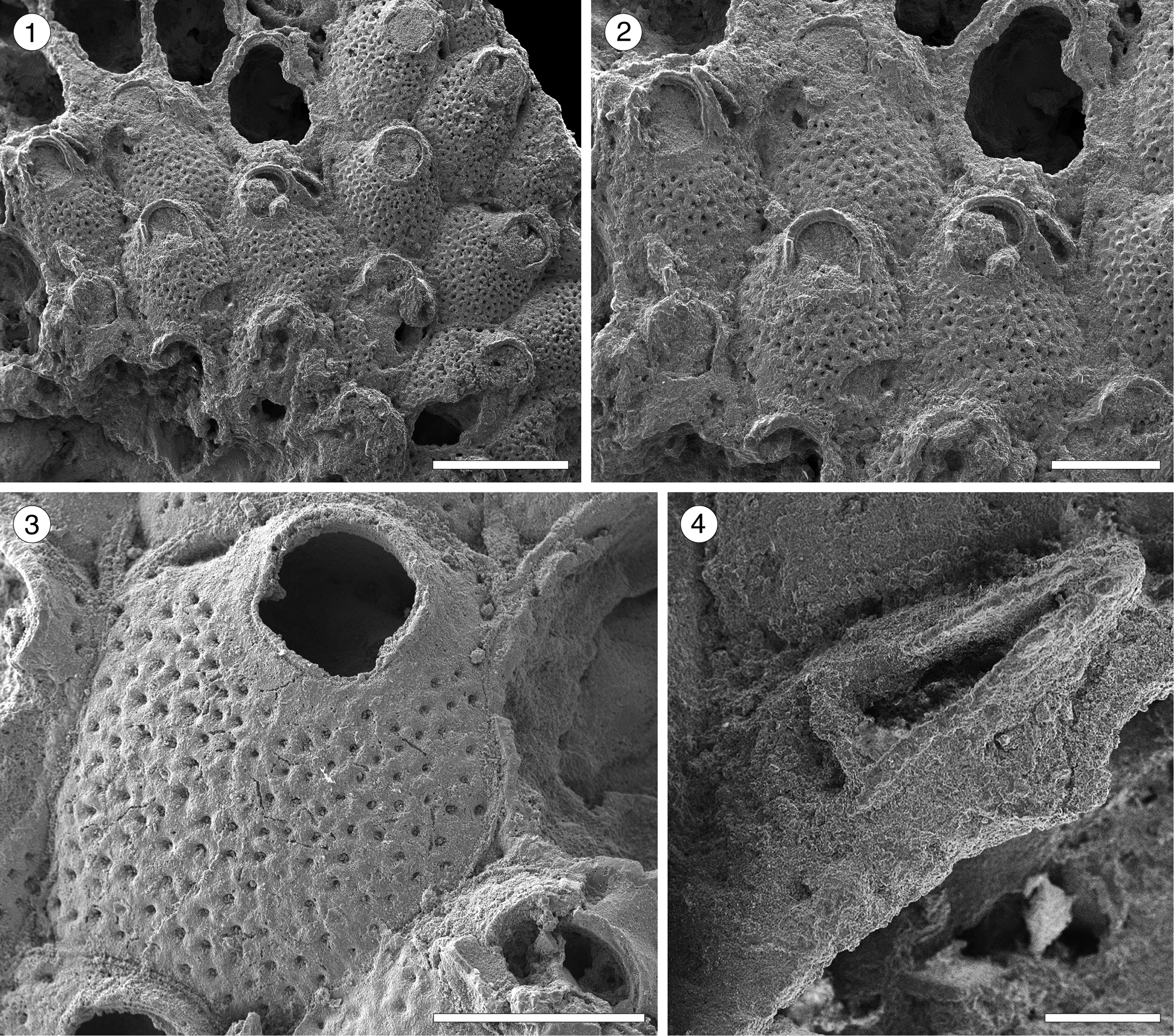
Figure 12. Hippopodina sp. indet. (MUN-STRI-47707). (1) General view of a colony fragment; (2) detail of the zooids bearing latero-oral avicularia; (MUN-STRI-47708) (3) detail of a zooid, with orifice showing a condyle; (4) detail of an avicularium with complete crossbar. All illustrated specimens are from the Siamaná Formation, Arroyo Ekieps. Scale bars are (1) 1 mm; (2, 3) 0.5 mm; (4) 0.1 mm.
Table 13. Measurements (in mm) of Hippodina sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Occurrence
Early Miocene, Siamaná Formation, Arroyo Ekieps, Colombia.
Description
Colony encrusting, multiserial, unilaminar. Autozooids separated by deep furrows and a narrow thread, rounded polygonal, longer than wide (mean L/W 1.38). Frontal shield convex, finely granular, evenly perforated by numerous (~130), circular pseudopores, ~0.02 mm in diameter. Larger, fusiform pores at the distal corners of the zooids sometimes visible. Orifice terminal, hoof-shaped, two robust condyles separating a semicircular anter from a shallow, broad sinus with proximal border flat or slightly concave. Adventitious avicularium single, sometimes absent, lateral to the orifice, originating at the same level as the orifice proximal margin and extending for the total length of the orifice; rostrum raised, narrow and acutely triangular, oriented distolaterally or medially; crossbar complete. Ovicells not observed.
Remarks
In the fossil record of North America two species of Hippopodina are known from the Eocene of Georgia and Florida (USA): H. stephensi Cheetham, Reference Cheetham1962b, and H. vibraculifera Canu and Bassler, Reference Canu and Bassler1917, respectively. The former species differs from Hippopodina sp. indet. in often having paired adventitious avicularia, placed and directed proximolaterally to the orifice, while the latter species has avicularia placed distally and oriented proximally.
In addition, five Recent species are known from off North America: H. pulcherrima (Canu and Bassler, Reference Canu and Bassler1928) from the Western Atlantic; H. bernardi Lagaaij, Reference Lagaaij1963, from the Gulf of Mexico; H. irregularis Osburn, Reference Osburn1940, from the Caribbean Sea; H. tahitiensis Leca and d’Hondt, Reference Leca and d'Hondt1993, allegedly from the Caribbean Sea, India, and Africa; and H. californica Osburn, Reference Osburn1952, from the Pacific coast of the USA. However, Hippopodina californica and H. irregularis lack avicularia; H. bernardi has a centrally imperforate frontal shield; H. tahitiensis bears single or paired, small, drop-shaped avicularia placed distolaterally and oriented distally; and H. pulcherrima, the most common in the Caribbean region (Tilbrook, Reference Tilbrook1999), has a narrower sinus and single or paired avicularia, located beside the orifice and oriented proximomedially.
Three species of Hippopodina are known from the early–middle Miocene of other geographic regions: H. lappi David, Reference David1965, from France lacks avicularia; H. emerensis Abbas and El-Senoussi, Reference Abbas and El-Senoussi1979, from Egypt has adventitious avicularia on either side of the orifice; and H. indicata Di Martino and Taylor, Reference Di Martino and Taylor2015, found in coral reefs from East Kalimantan, has similar adventitious avicularia but originating more proximally and with the rostrum pointing to the orifice condyles. Our specimens resemble the Recent species H. iririkiensis Tilbrook, Reference Tilbrook1999, recorded in the Indo-West Pacific, Australia, and the Mediterranean Sea in the shape, location, and direction of the single avicularium; however, in H. iririkiensis, avicularia are often paired, Hippopodina sp. indet. has larger zooids (1.12 × 0.82 mm vs. 0.9 × 0.62 mm), and the proximal margin of the orifice is flatter. However, the limited amount of material available and the lack of ovicells in our specimens prevented us from confirming its conspecificity with previously described species and from describing a new species. In the Siamaná Formation, Hippopodina sp. indet. was found encrusting the coral species Porites anguillensis and Millepora sp., co-occurring with Poricella paulae n. sp.
Family Teuchoporidae Neviani, Reference Neviani1895a
Genus Cycloavicularia new genus
Type species
Cycloavicularia parva n. sp., from Arroyo Ekieps, Colombia, early Miocene, Siamaná Formation; by monotypy.
Diagnosis
As for the type species.
Etymology
From the Latinized form of the Greek kyklos, meaning circle, plus the morphologic term avicularia, alluding to the circular, adventitious avicularia placed on the zooidal ‘shoulders.’
Remarks
We place this new genus in the family Teuchoporidae based on the following characters: frontal shield evenly perforated, peristome moderately high, presence of small adventitious avicularia, and a conspicuous and perforated ooecium that is proximally encroached by the peristome (Harmer, Reference Harmer1957; Poluzzi, Reference Poluzzi1977; Gordon, Reference Gordon1984). This family includes three genera: Teuchopora Neviani, Reference Neviani1895b (type), “Coleopora” Canu and Bassler, Reference Canu and Bassler1927, and Lagenicella Cheetham and Sandberg, Reference Cheetham and Sandberg1964. Cycloavicularia n. gen. differs from Teuchopora and “Coleopora” in having adventitious avicularia while lacking the proximal peristomial denticle. It differs also from Lagenicella in having polygonal rather than vase-shaped zooids, and in the lack of imperforate, hood-like projections rimming the peristomial ovicells. Cycloavicularia n. gen. also shows some similarities with members of the family Exechonellidae Harmer, Reference Harmer1957, which, however, usually lack or have inconspicuous ovicells (Gordon, Reference Gordon1984).
The type species of this new genus closely resembles the Recent Brazilian species Marcusadorea pinheroi Almeida et al., Reference Almeida, Souza, Menegola and Vieira2017, in its overall appearance, size of the zooids and ovicells, and density and distribution of pseudopores, but differs in having avicularia. Its allocation in Marcusadorea, however, would not be justified because Marcusadorea jamaicensis Vieira, Migotto, and Winston, Reference Vieira, Migotto and Winston2010, the type species of the genus and family Marcusadoreidae Winston, Vieira, and Woollacott, Reference Winston, Vieira and Woollacott2014, has an irregularly perforated frontal shield, marginal areolar pores are distinct from the pseudopores, and peristomial avicularia are sometimes present (Vieira et al., Reference Vieira, Migotto and Winston2010), while Cycloavicularia parva n. gen. n. sp. has an evenly perforated frontal shield and distinctive, small, adventitious avicularia in the distolateral corners of the zooids.
Cycloavicularia parva new species
Figure 13; Table 14
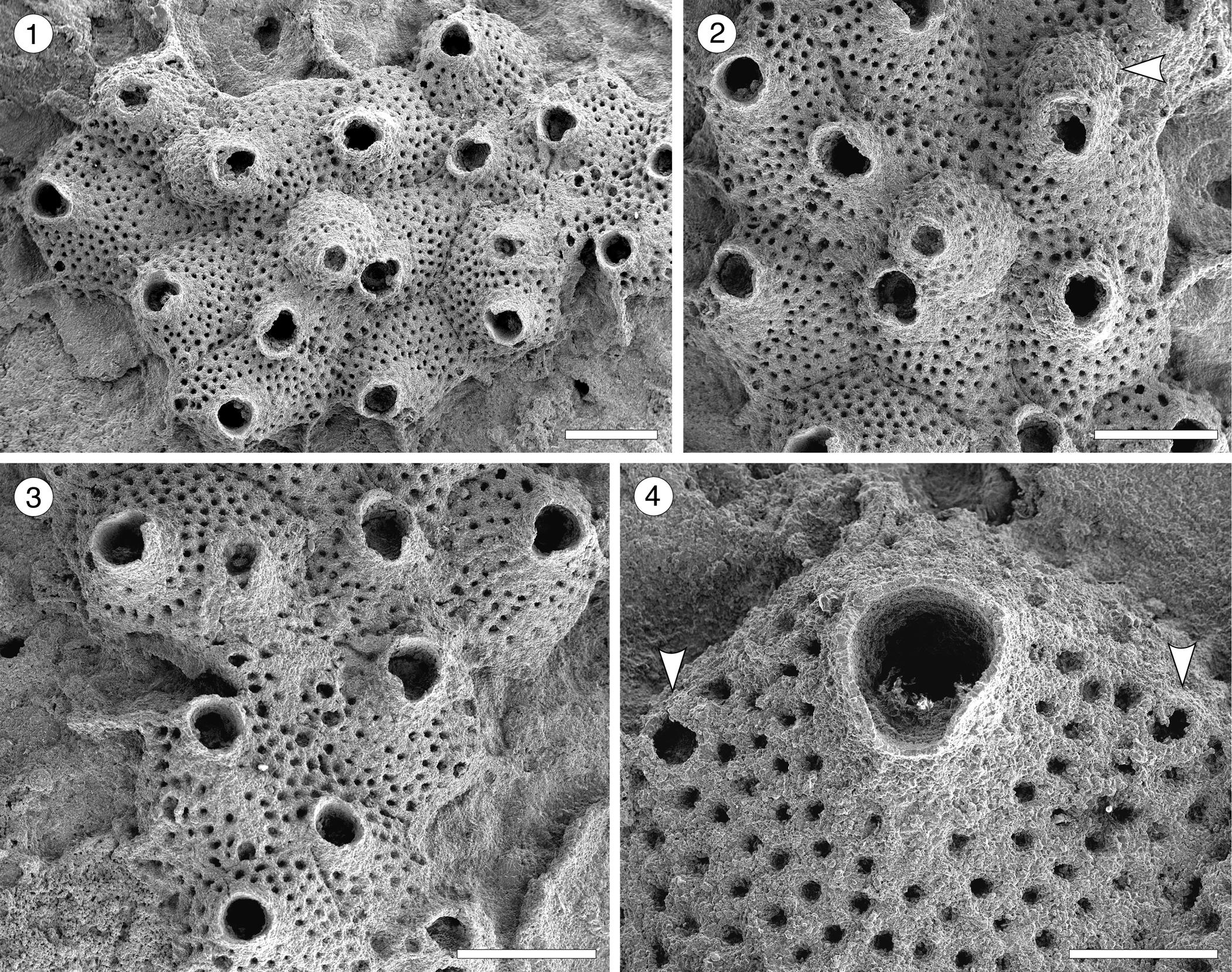
Figure 13. Cycloavicularia parva n. gen. n. sp. (Holotype MUN-STRI-47709) from the Siamaná Formation, Arroyo Ekieps. (1) General view of the colony; (2) detail of a group of recumbent zooids and a fertile zooid (arrowed); (3) detail of the peristome and sinus of the secondary orifice; (4) detail of the rounded avicularia (arrowed). Scale bars are (1–3) 0.5 mm; (4) 0.2 mm.
Table 14. Measurements (in mm) of Cycloavicularia parva n. gen. n. sp. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Holotype
MUN-STRI-47709, from the lower Miocene Siamaná Formation, Arroyo Ekieps, La Guajira, Colombia.
Diagnosis
Colony encrusting. Orifice surrounded by a well-developed, imperforate peristome, taller distolaterally, forming an U-shaped peristomial sinus proximally. Frontal shield granular, evenly pseudoporous except for the peristome. Two small, circular, adventitious avicularia placed at distolateral corners of autozooids. Ovicell globular; ooecium granular and pseudoporous as the frontal shield, encroached proximally by the peristome.
Description
Colony encrusting, multiserial, uni- to multilaminar. Autozooids large, distinct by narrow grooves, recumbent to semi-erect, rhomboidal to irregularly polygonal, slightly longer than wide (mean L/W 1.15). Frontal shield slightly convex, granular, evenly perforated, except for the peristome, by 20–100 (depending on the length of the zooid) circular pseudopores ~0.03 mm in diameter; occasionally 2–3 rows of marginal pores distally. Orifice terminal to subterminal; primary orifice hidden by a distolaterally well-developed peristome forming a secondary orifice with U-shaped sinus; secondary orifice slightly larger in ovicellate zooids. Adventitious avicularia small, circular, placed on the distolateral corners of the autozooids; crossbar or pivotal condyles not preserved. Ovicell produced by the distal zooid; hyperstomial, globular, opening into the peristome (acleithral); ooecium surface granular and pseudoporous as the frontal shield. Ancestrula unknown.
Etymology
From the Latin parvus, meaning little, in reference to the reduced size of the adventitious avicularia.
Remarks
Cycloavicularia parva n. gen. n. sp. resembles the Indo-Pacific Recent species Cosciniopsis lonchaea (Busk, Reference Busk1884) in having a well-developed peristome, a tuberculate and pseudoporous frontal shield, and adventitious avicularia, but the latter species has one or two triangular avicularia close to the orifice, conspicuous condyles, lateral walls with uniporous septula, and ovicells that are closed by the operculum (cleithral) (Tilbrook, Reference Tilbrook2006, p. 236–237, pl. 52, figs. a–c). Species of the genus Saevitella Bobies, Reference Bobies1956, also have granular and pseudoporous frontal shields and ovicells, but lack avicularia and have cleithral ooecia (Berning, Reference Berning2012, p. 43, figs. 13–18). In the Siamaná Formation, Cycloavicularia parva n. gen. n. sp. was found encrusting cavities of the coral Porites anguillensis.
Superfamily Celleporoidea Johnston, Reference Johnston1838
Family Phidoloporidae Gabb and Horn, Reference Gabb and Horn1862
Genus Pleuromucrum Vigneaux, Reference Vigneaux1949
Type species
Pleuromucrum saucatsense Vigneaux, Reference Vigneaux1949, from Saucats (Pont-Pourquey), France, Burdigalian; by original designation.
Pleuromucrum sp. indet.
Figure 14; Table 15
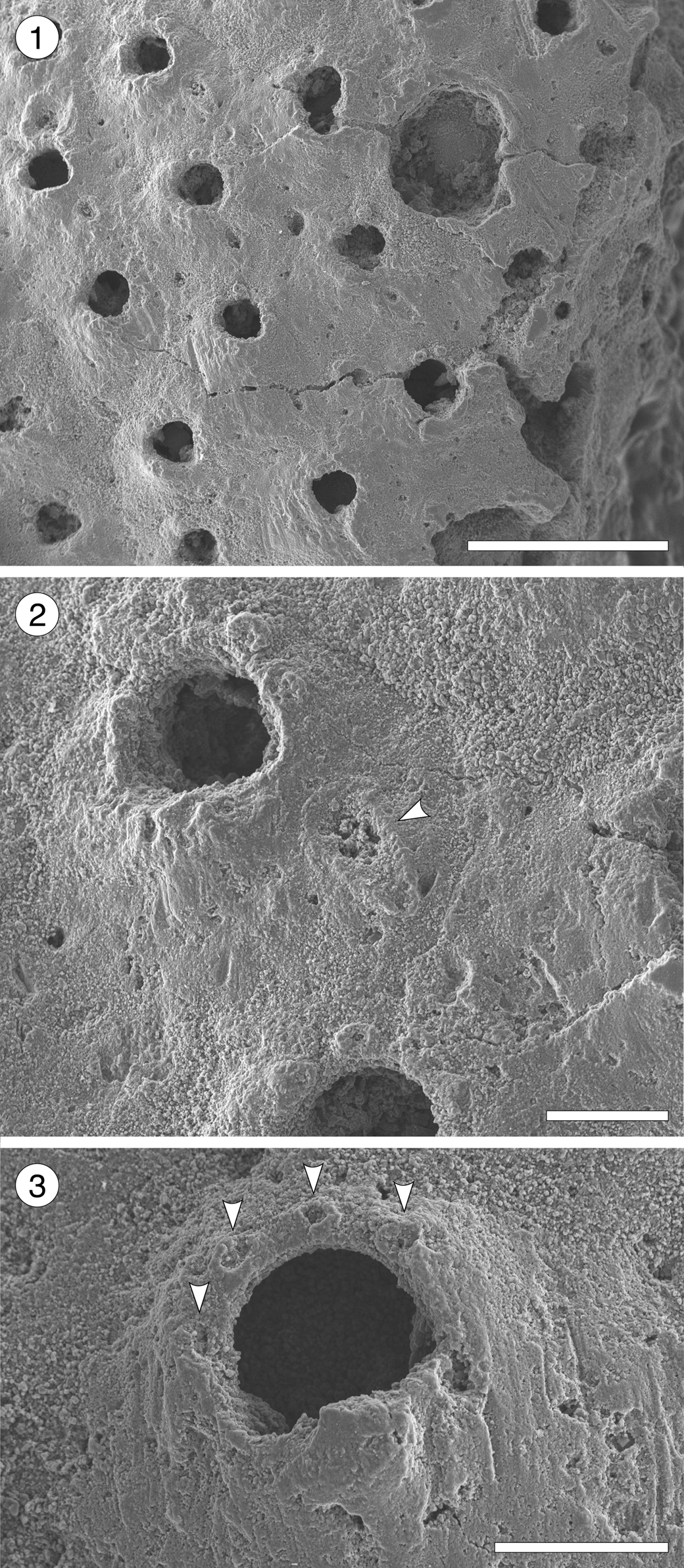
Figure 14. Pleuromucrum sp. indet. (MUN-STRI-47710) from the Siamaná Formation, Arroyo Uitpa. (1) General view of a colony fragment; (2) detail of a zooid bearing a triangular avicularium (arrowed); (3) detail of the orifice showing four oral spine bases (arrowed), condyles, and suboral umbo. Scale bars are (1) 0.5 mm; (2, 3) 0.1 mm.
Table 15. Measurements (in mm) of Pleuromucrum sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Occurrence
Early Miocene, Siamaná Formation, Arroyo Uitpa, Colombia.
Description
Colony encrusting, multiserial, unilaminar. Autozooids almost indistinct, boundaries apparently concealed by secondary calcification, subrhomboidal to oval, slightly longer than wide (mean L/W 1.19). Frontal shield smooth, generally slightly depressed, but raised suborally, imperforate except for 2–4 small marginal areolar pores. Orifice bell-shaped, almost as long as wide, bearing two robust, triangular condyles; four distolateral oral spine bases; proximal border with a poorly developed or poorly preserved umbo. Adventitious avicularium single, placed on one side of the frontal shield slightly below the orifice; rostrum rounded triangular, directed proximolaterally, seemingly with pivotal condyles. Ooecium broken, apparently small and circular in outline.
Remarks
We place this specimen in Pleuromucrum based on the shape of the orifice, the imperforate frontal shield with few, sparse marginal pores, the presence of frontal triangular avicularia, oral spines and suboral umbo, and the proportionally small ooecium in respect to autozooid size (Di Martino and Taylor, Reference Di Martino and Taylor2017). The smooth appearance of the frontal shield (in Pleuromucrum it is usually nodular) is likely due to preservation, which also prevents species-level assignment. In the Siamaná Formation, Pleuromucrum sp. indet. was found encrusting coralline algae.
Genus Reteporellina Harmer, Reference Harmer1933
Type species
Retepora denticulata Busk, Reference Busk1884, from Honolulu, Hawaii, North Pacific, Recent; by original designation.
Reteporellina sp. indet.
Figure 15; Table 16
Figure 15. Reteporellina sp. indet. from the Siamaná Formation, Arroyo Ekieps: (MUN-STRI- 47712). (1) General view of the branch fragment showing three rows of zooids and the areolar pores (arrowed); (2) detail of the sinus in the peristome (arrowed); (3) detail of the bifid suboral avicularium (arrowed); (4) detail of the medial suture of the ovicell (arrowed); (5) detail of the vibices of the abfrontal surface. Scale bars are (1, 2) 0.2 mm; (3, 4) 0.1 mm; (5) 0.5 mm.
Table 16. Measurements (in mm) of Reteporellina sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Occurrence
Early Miocene, Siamaná Formation, Arroyo Ekieps, Colombia.
Description
Colony erect, rigid. Branches cylindrical to slightly flattened, 0.32–0.50 mm wide. Autozooids distinct, separated by raised ridges, subrhomboidal, arranged alternately in three longitudinal rows on the frontal side of the branch, more than twice as long as wide (mean L/W 2.10). Primary orifice hidden by a proximally well-developed peristome with a U-shaped sinus medially. Frontal shield slightly convex, perforated by a pair of marginal areolar pores placed proximally at about one-third of the total length of the zooid. Adventitious avicularia large, placed on a suboral, raised cystid; rostrum bifid, oriented latero-frontally. Ovicell hyperstomial, globular, longer than wide, seemingly with a medial suture. Abfrontal surface smooth with irregular vibices; no avicularia or pores observed.
Remarks
Seven Recent species of Reteporellina are known in America: R. marsupiata (Smitt, Reference Smitt1873) from the West Atlantic; R. prominens (Canu and Bassler, Reference Canu and Bassler1928) from the Gulf of Mexico; R. directa Winston and Woollacott, Reference Winston and Woollacott2009, from Barbados; R. evelinae Marcus, Reference Marcus1955, from Brazil; R. moyanoi d’Hondt, Reference d'Hondt1981, from Uruguay and the Pacific coast; R. bilabiata Osburn, Reference Osburn1952, from the Gulf of California; and R. denticulata gracilis Osburn, Reference Osburn1952, from Ecuador and Costa Rica. Among them, R. directa and R. moyanoi differ from Reteporellina sp. indet. in having the frontal avicularia oriented proximally not latero-frontally. Reteporellina prominens and R. denticulata gracilis have small frontal avicularia that are rounded and drop-shaped, respectively; R. bilabiata has frontal avicularia with a triangular rostrum, placed proximolateral to the orifice, and avicularia also on the abfrontal surface. Reteporellina sp. indet. closely resembles R. evelinae and R. marsupiata in the arrangement of zooids and in having frontal avicularia with bifid rostra. However, poor preservation of the specimen prevents any further comparison. In the Siamaná Formation, Reteporellina sp. indet. was found in sediment adhering to the corals Acropora panamensis and Caryophyllidae sp., co-occurring with Catenicella sp., Licornia sp., Margaretta cf. M. buski, and Ditaxiporina colombiana n. sp.
Genus Rhynchozoon Hincks, Reference Hincks1895
Type species
Lepralia bispinosa Johnston, Reference Johnston1847, from Berwick Bay, United Kingdom, North Sea, Recent; by original designation.
Rhynchozoon sp. indet.
Figure 16; Table 17
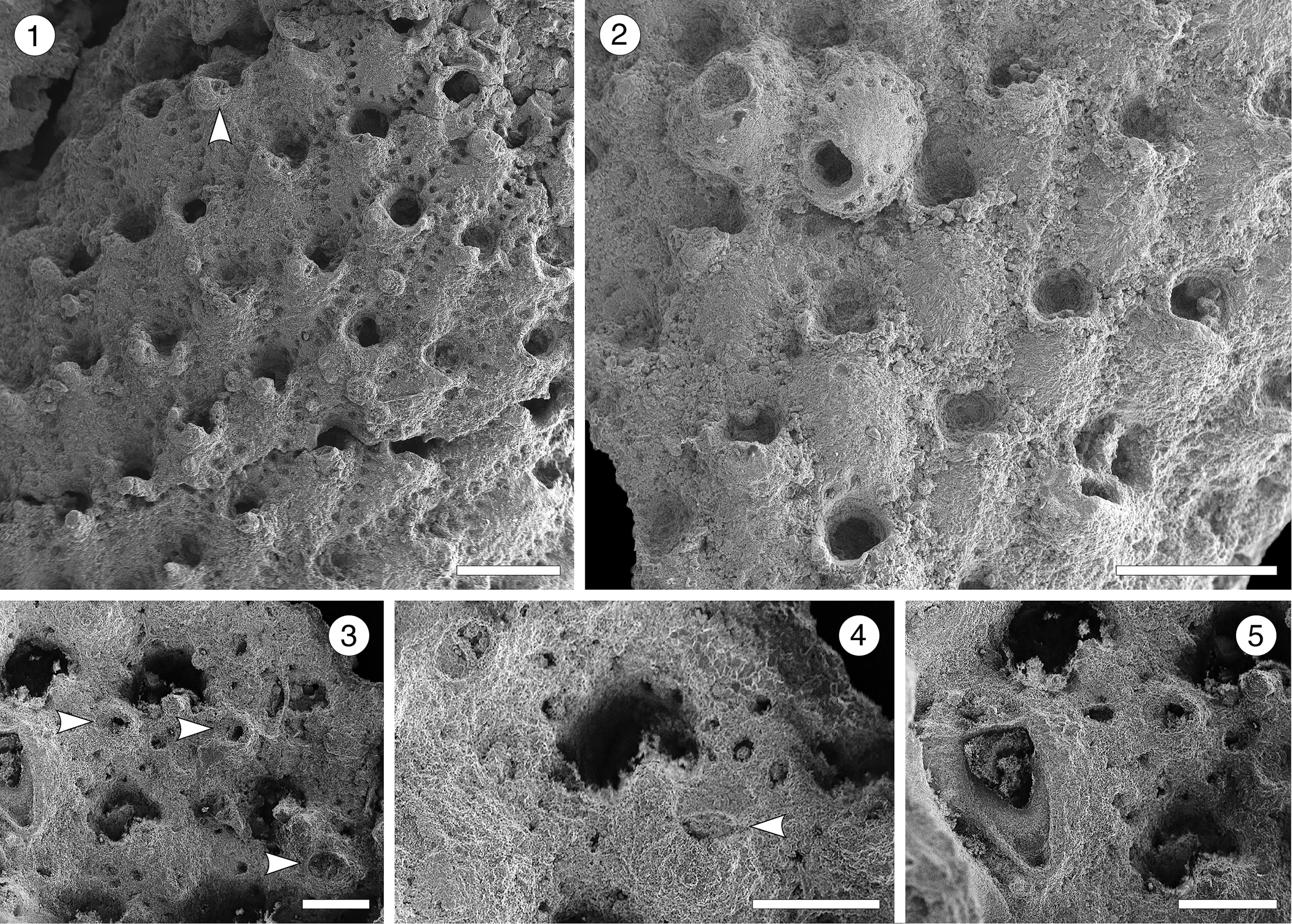
Figure 16. Rhynchozoon sp. indet. from the Siamaná Formation, Arroyo Ekieps: (MUN-STRI-47713). (1) General view of the colony fragment, and suboral avicularia (arrowed); (2) group of zooids, some of them showing the primary subcircular orifice; (3) detail of erect zooids and rounded avicularia (arrowed); (4) detail of the secondary orifice showing the suboral avicularium (arrowed); (5) detail of the triangular interzooidal avicularium. Scale bars are (1, 2) 0.5 mm; (3–5) 0.2 mm.
Table 17. Measurements (in mm) of Rhynchozoon sp. indet. X = mean; R = observed range; SD = standard deviation; N = number of measurements.
Description
Colony encrusting, multiserial, uni- to multilaminar. Autozooids at the colony growing edge, distinct, oval to claviform, longer than wide (mean L/W 1.82), but indistinct, erect to semi-erect, and irregularly arranged in central, older areas. Frontal shield convex, smooth to slightly ribbed, imperforate except for 14–16 circular to drop-shaped marginal areolar pores (0.03 mm in diameter) separated by ridges. Orifice subcircular. Suboral mucro bearing apically a small, rounded avicularium with complete crossbar, rostrum oriented laterally. Often, two additional, raised hooked processes flanking the medial mucro. Dimorphic interzooidal avicularia placed between the erect zooids: either large, triangular, placed proximolateral to the orifice and oriented proximally, or elliptical and oriented proximolaterally; both types with complete crossbar. Ovicells not observed.
Remarks
Based on its general appearance, including the arrangement of zooids, the well-developed suboral processes, and the monomorphic suboral avicularia, we assign these specimens to Rhynchozoon. However, the poor preservation prevents the comparison with congeners and nomenclature remains open. In the Siamaná Formation, Rhynchozoon sp. indet. was found encrusting the hydrocoral Millepora alcicornis Linnaeus, Reference Linnaeus1758, and the scleractinian Colpophyllia willoughbiensis, co-occurring with Calpensia caribensis and Copidozoum sp. indet.
Discussion
The study area comprises patch reefs distributed in a shallow lagoon and in the discontinuous coral barrier enclosing the lagoon (Flórez, Reference Flórez2020). These coral communities thrived in tropical waters, at depths of 2–30 m, with low turbidity, low energy, and limited siliciclastic input (Flórez et al., Reference Flórez, Zapata-Ramírez and Klaus2019a, Reference Flórez, Zapata-Ramírez and Klausb; Flórez, Reference Flórez2020). Associated with them, we found 32 bryozoan species (Flórez et al., Reference Flórez, Di Martino and Ramalho2021; this paper).
New species account for 31% (10 species) of the whole assemblage. Three new species belong to the family Cribrilinidae, and one each to Antroporidae, Microporidae, Onychocellidae, Steginoporellidae, Catenicellidae, Arachnopusiidae and Teuchoporidae. The new genera, Atoichos Flórez et al., Reference Flórez, Di Martino and Ramalho2021, in Onychocellidae; Gymnophorella Flórez et al., Reference Flórez, Di Martino and Ramalho2021, in Steginoporellidae; and Cycloavicularia n. gen. in Teuchoporidae, also have been introduced to accommodate three new species.
Two species are identified as confer and another three as affinis to preexisting species. The remaining species (53%) were left in open nomenclature, one identified at family level and 15 at genus level. This high percentage of undetermined species is due to the poor preservation caused by weathering, dissolution and recrystallization, and/or mechanical abrasion, which resulted in the loss or alteration of key skeletal features. It is well known that tropical Cenozoic carbonates, especially coral reefs, experience rapid cementation (Macintyre, Reference Macintyre and Hopley2011), and severe diagenetic alterations greatly affecting aragonitic organisms, even to the complete disappearance of the aragonitic component. Taylor and Di Martino (Reference Taylor and Di Martino2014) found that 27% of tropical cheilostome species encrusting the underside of platy corals were aragonitic and 30% bimineralic. These results suggest that species richness may suffer a great loss in this tropical paleoenvironment, although it has also been observed that in some particular conditions the originally aragonitic skeletons of bryozoans can be preserved by calcitization (Di Martino et al., Reference Di Martino, Taylor, Kudryavtsev and Schopf2016). Furthermore, the preservation of bryozoan colonies may also be affected by the diagenetic processes affecting their aragonitic substrates, such as scleractinian corals (as in this case) or mollusks (Taylor and Di Martino, Reference Taylor and Di Martino2014).
The relative proportions of cyclostomes (6%) and cheilostomes (94%) follow the general pattern observed in the Caribbean region from the Miocene to the Pleistocene (Cheetham et al., Reference Cheetham, Jackson, Sanner and Ventocilla1999; Taylor, Reference Taylor2001), and in Recent coral-associated bryozoan faunas (Winston, Reference Winston1986).
In the Siamaná Formation, 75% of species are encrusting and 24% erect (18% articulated and 6% rigid). These proportions are similar in Recent communities and in some fossil assemblages in tropical coral reef environments (Winston, Reference Winston1986; Cheetham and Jackson, Reference Cheetham, Jackson, Herrera-Cubilla and Jackson2000; Di Martino et al., Reference Di Martino, Taylor and Johnson2015). The principal substrates encrusted by bryozoans were the scleractinian corals Alveopora tampae, Acropora panamensis, Colpophyllia willoughbiensis, Goniopora hilli, Porites anguillensis, Porites baracoaensis, Acropora sp., Porites sp., Caryophyllidae gen. et sp. indet., the hydrocoral Millepora sp., as well as coralline algae and mollusk shells.
Species richness varies greatly among the three sampling localities: 75% of species were found in Arroyo Ekieps, 25% in Uitpa, and 3% in Flor de La Guajira (Appendix 2). The highest species richness in Arroyo Ekieps is associated with a higher structural complexity compared to the small patch reefs in Arroyo Uitpa and Flor de La Guajira. A single species, Hippopleurifera sp. indet. 1, was found in Flor de La Guajira, and was also present in Arroyo Uitpa. The remaining species are exclusive to each locality.
Except for the three newly introduced genera, Atoichos, Gymnophorella (Flórez et al., Reference Flórez, Di Martino and Ramalho2021), and Cycloavicularia n. gen., the genera found in the Siamaná Formation have been observed previously in the Great Caribbean Region, Gulf of Mexico, and north of Brazil, either exclusively in the fossil record or contemporary environments, or both. For eight genera, the age range was extended back to the early Miocene (Aquitanian) (Fig. 17). Glabrilaria stands-out among these, because it has only recently been found in the Caribbean region associated with Recent deep-sea coral banks (Rosso et al., Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018) and off the Amazon River mouth in bryozoan reefs (Ramalho et al., Reference Ramalho, Moraes, Salgado, Bastos and Moura2021).
Figure 17. Range chart of the first and last occurrences in the greater Caribbean region, Gulf of Mexico, and Brazil, of the genera reported here and in Flórez et al. (Reference Flórez, Di Martino and Ramalho2021); asterisks indicate the genera whose ranges have been extended to the early Miocene. The striped interval is the estimated stratigraphic range for the reefs of the Siamaná Formation (ca. 23–20.5 Ma). References: Canu and Bassler (Reference Canu and Bassler1917, Reference Canu and Bassler1920, Reference Canu and Bassler1923); Cheetham (Reference Cheetham1962a, Reference Cheethamb, Reference Cheetham1963); Winston (Reference Winston1984, Reference Winston1986, Reference Winston2005); Winston and Cheetham (Reference Winston, Cheetham, Eldredge and Stanley1984); Cheetham et al. (Reference Cheetham, Jackson, Sanner and Ventocilla1999, Reference Cheetham, Sanner and Jackson2007); Taylor (Reference Taylor2001); Taylor and McKinney (Reference Taylor and McKinney2006); Flórez et al. (Reference Flórez, Montoya-Cadavid, Reyes-Forero and Santodomingo2007, Reference Flórez, Di Martino and Ramalho2021); Montoya-Cadavid et al. (Reference Montoya-Cadavid, Flórez and Winston2007); Di Martino et al. (Reference Di Martino, Taylor and Portell2017, Reference Di Martino, Taylor and Portell2019); Rosso et al. (Reference Rosso, Beuck, Vertino, Sanfilippo and Freiwald2018); Martha et al. (Reference Martha, Taylor and Rader2019); Ramalho et al. (Reference Ramalho, Serrano, Rueda, Távora and Zágoršek2019, Reference Ramalho, Moraes, Salgado, Bastos and Moura2021).
The Neogene bryozoan fauna associated with shallow-water paleoenvironments from the Gulf of Mexico and the Caribbean Basin is relatively well studied (Cheetham et al., Reference Cheetham, Jackson, Sanner and Ventocilla1999; Di Martino et al., Reference Di Martino, Jackson, Taylor and Johnson2018). In other tropical regions from South America, such northern Brazil, studies are incipient and knowledge of early Miocene bryozoans is partial (Zágoršek et al., Reference Zágoršek, Ramalho, Berning and Távora2014; Ramalho et al., Reference Ramalho, Távora, Tilbrook and Zágoršek2015, Reference Ramalho, Serrano, Rueda, Távora and Zágoršek2019; Aguilera et al., Reference Aguilera, Bencomo, de Araújo, Dias, Coletti, Lima, da Silva-Caminha, Polk, Alves-Martins, Jaramillo, Kutter and Lopes2020). However, compared to these faunas, in which erect and free-living species are dominant (Cheetham and Jackson, Reference Cheetham, Jackson, Jackson, Budd and Coates1996; Ramalho et al., Reference Ramalho, Távora, Tilbrook and Zágoršek2015, Reference Ramalho, Serrano, Rueda, Távora and Zágoršek2019; Di Martino et al., Reference Di Martino, Jackson, Taylor and Johnson2018), the Siamaná bryozoan assemblage shows a different ecological pattern and taxonomic composition at the genus level. In addition to the assemblage being older (Aquitanian), with the exception of the Brazilian fauna, these differences may be attributed to the reefal paleoenvironment, which favors cryptic, encrusting species colonizing the coral framework, as also observed in other tropical regions (Di Martino and Taylor, Reference Di Martino and Taylor2014, Reference Di Martino and Taylor2015). Nonetheless, genera such as Margaretta and Nellia are present throughout the regions over time.
Compared to other bryozoan faunas associated with Miocene coral reefs, the species richness in the Siamaná Formation is significantly lower (e.g., 123 species were reported from Indonesia [Di Martino and Taylor, Reference Di Martino and Taylor2014, Reference Di Martino and Taylor2015] and 56 species from the Mediterranean [Hamdane and Moissette, Reference Hamdane, Moissette, Wyse-Jackson, Buttler and Spencer-Jones2002]). These differences may be an effect of sampling effort in fresh exposures. In addition, the greater bryozoan diversity of the Indonesian reefs can be explained by the high input of siliciclastic sediment that facilitates the preservation of the material (Di Martino et al., Reference Di Martino, Taylor and Johnson2015), including the calcitization of originally aragonitic taxa (Di Martino et al., Reference Di Martino, Taylor, Kudryavtsev and Schopf2016).
The bryozoan fauna of the Siamaná Formation highlights the remarkable diversity of cryptic species in the ancient coral reefs of the Southern Caribbean region.
Acknowledgments
We thank P. Zapata for collecting the coral samples (Colciencias Project 727756933195); J.C. Braga and C. Jaramillo for their comments and support; R. Cuffey and P. Bock for helping with the bibliography; J. Souto, C. López-Fé, and L.M. Vieira for taxonomic advice; Corporación Geológica ARES for the logistics and the Wayúu community for the guide in field. We are grateful for the support of Ecopetrol S.A., STRI, University of Zurich, Universidad del Norte, NSF (Grant EAR 0957679), National Geographic Society, Anders Foundation, 1923 Fund, and G.D. and J. Walston Johnson during the expeditions. The research group of the Junta de Andalucía RNM 190 supported SEM work and the Universidad de Granada the open access fees. PF was funded by the Colciencias scholarship Doctorados en el Exterior 728. EDM was funded by the Research Council of Norway grant 314499. We are grateful to the reviewers, J. López-Gappa and B. Berning, and to the Associate Editor, P.D. Taylor, for their valuable comments that greatly improved the first submitted version of this manuscript.
Appendices
Appendix 1. Localities, stations and coordinates of the Siamaná Formation in the studied area. Geologic ages inferred from strontium isotopes in coralline algae (Silva-Tamayo et al., Reference Silva-Tamayo, Lara, Nana Yobo, Erdal, Sanchez and Zapata-Ramírez2017), and faunistic assemblages found in the studied outcrops (Flórez et al., Reference Flórez, Di Martino and Ramalho2021).

Appendix 2. List of bryozoans found in the Siamaná Formation (Cocinetas Basin in La Guajira Peninsula, Colombia). The collection is hosted at the Mapuka Museum of the Universidad del Norte, Barranquilla-Colombia. Bryozoan colony attached to the coral (*).