Sommer et al (Reference Sommer, Aleman and Ramsey2001) used meta-analyses to review the literature on handedness, dichotic listening and anatomical asymmetry in schizophrenia and concluded that there was strong evidence for decreased cerebral lateralisation. As they point out, the implication is that finding the locus of the gene for cerebral dominance could unravel the genetic predisposition to schizophrenia. Procopio (Reference Procopio2001) raises a number of points relevant to the findings of Sommer et al and concludes that both genetic and environmental factors have to be accounted for — ‘the right shift is still only a hypothesis’. Procopio's comments focus on handedness and it is important to remind ourselves, as Sommer et al's review makes clear, that handedness is no more than an indirect and developmentally labile index of anatomical asymmetry and language lateralisation; it is this last variable and its genetic determination (Crow, Reference Crow1998a ,Reference Crow b ) that is of greatest relevance.
GENETIC AND ENVIRONMENTAL INFLUENCES
Procopio (Reference Procopio2001) rightly draws attention to the study by Steinmetz et al (Reference Steinmetz, Herzog and Schlaug1995) of asymmetry of the planum temporale in monozygotic twins concordant and discordant for handedness. Twins discordant for handedness were more likely to be discordant for planum temporale asymmetry (Fig. 1). Handedness and asymmetry are thus related, but if the underlying determinant is genetic why should monozygotic twins be discordant, as they often are, for either? Procopio proposes that an environmental influence is relevant but the nature of such an influence is obscure (see below). The alternative is to assume that there are unaccounted for (random or epigenetic) variations in development. The importance of the study of Steinmetz et al is that it demonstrates that such presently intangible variation is large in relation to the component that, according to a simple view, can be attributed to sequence variation (i.e. that common to identical twins), as has always been implicit in Annett's (Reference Annett1999) right-shift theory.
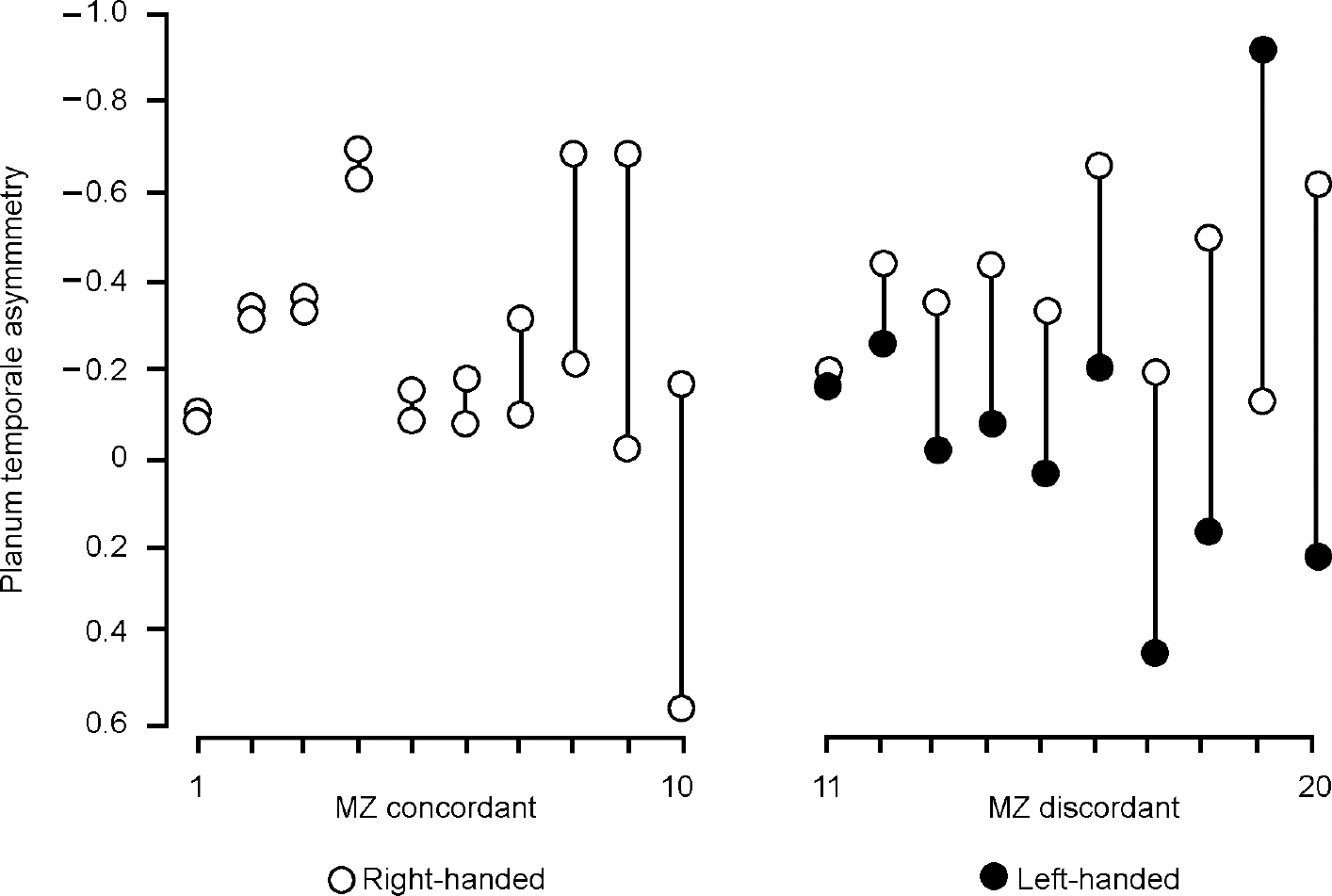
Fig. 1 Distribution of direction and degree of planum temporale asymmetry in 20 pairs of monozygotic (MZ) twins. MZ concordant: twin pairs 1-10 concordant for handedness; MZ discordant: twin pairs 11-20 discordant for handedness (X-axis). Y-axis: negative values indicate left-ward, positive values right-ward planum temporale asymmetry (adapted from Reference Steinmetz, Herzog and SchlaugSteinmetz et al, 1995).
What environmental factor might influence the development of cerebral asymmetry? Procopio points to the finding of Salvesen et al (Reference Salvesen, Vatten and Eik-Nes1993) that children who had been screened by ultrasonography in utero were more likely to be non-right-handed than those who had not. However, the difference was small (odds ratio 1.32%; 95% CI 1.02-1.71). As Salvesen et al emphasise, non-right-handedness was one of six initial hypotheses, and no association with impaired neurological development was found. An effect of ultrasound on cerebral dominance is a concern but the evidence is modest.
NATURE AND LOCATION OF THE ASYMMETRY FACTOR
The key question is the nature of the asymmetry factor itself. Unless this can be identified as more than a hypothetical gene, not much progress can be expected. That it is the key to understanding disorders of development was suggested by Orton (Reference Orton1937) in relation to reading disability, and Annett (Reference Annett1985) in relation to cognitive development in general. Orton predicted deficits at the point of equal hand skill and Annett, on the basis of heterozygote advantage formulation, at the extremes of the hand skill continuum (see Reference AnnettAnnett, 1999). Our findings in the National Child Development Study cohort (Crow et al, Reference Crow, Done and Sacker1996, Reference Crow, Crow and Done1998; see also Reference Leask and CrowLeask & Crow, 2001), including that reading disability is a precursor of psychosis (Reference Crow, Done and SackerCrow et al, 1995), support Orton more strongly than Annett, but the important point is that the dimension of lateralisation is a determinant of the human ability to handle symbols. Recent evidence reinforces the conclusion that directional asymmetry on a population basis is a human (or at least hominid) characteristic (Fig. 2). What this evidence suggests is that at some point in hominid evolution there was a discrete genetic change (a saltation), and that this change was associated with cerebral lateralisation and played a role in the evolution of language. This hypothesis can be related to the argument (e.g. Reference BickertonBickerton, 1995) that language is a relatively recent and abrupt acquisition in the hominid lineage and to evidence (e.g. Reference MellarsMellars, 1998) from the archaeological records that the ability to represent in symbols goes back no more than 90 000 years. These general views are consistent with the concept (Reference Stringer and McKieStringer & McKie, 1996) that the capacity for language is the characteristic that defines modern Homo sapiens as a species with an origin somewhere in East Africa around 100 000 years ago. The question is unresolved regarding whether lateralisation was introduced at this time, or whether it was present earlier, for example in Homo erectus (Reference SteeleSteele, 1998) and was modified by a subsequent genetic change. The implication of this evolutionary perspective is that the genetic changes that led to the evolution of language were relatively simple and small in number.
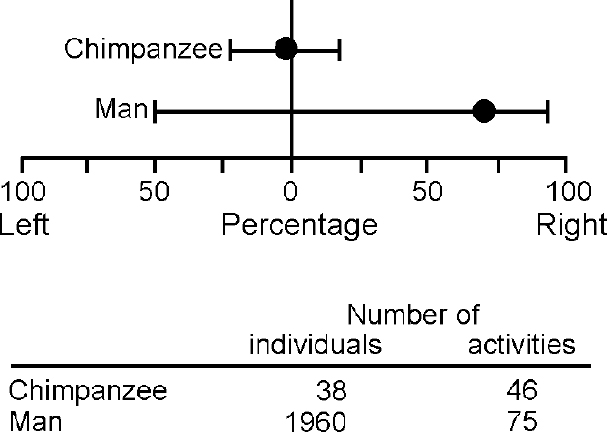
Fig. 2 Direction of hand preference for a range of daily activities in a population of Homo sapiens (data from Provins et al, Reference Provins, Milner and Kerr1982) and Pan troglodytes in the Gombe National Park (data from Reference Marchant and McGrewMarchant & McGrew, 1996). Each individual is assigned a point on the 100% left to 100% right scale. Medians and boundary values (horizontal bars) for 95% of the populations have been calculated from data on graphs in the original publications.
I have argued (Crow, Reference Crow1993, Reference Crow1994) that the pattern of verbal and spatial deficits associated with the sex chromosome aneuploidies indicates that the genetic determinant of asymmetry is in a region of homology between the X and the Y chromosomes. The evolutionary history of the sex chromosomes provides a pointer to its location (Reference Lambson, Affara and MitchellLambson et al, 1992; Sargent et al, Reference Sargent, Briggs and Chalmers1996, Reference Sargent, Boucher and Blanco2001; Reference Schwartz, Chan and BrownSchwartz et al, 1998): after the separation of the lineages that led to the chimpanzee and H. sapiens a translocation occurred from Xq21.3 to the Y chromosome short arm, and the translocated block was split by a subsequent paracentric inversion (Fig. 3).
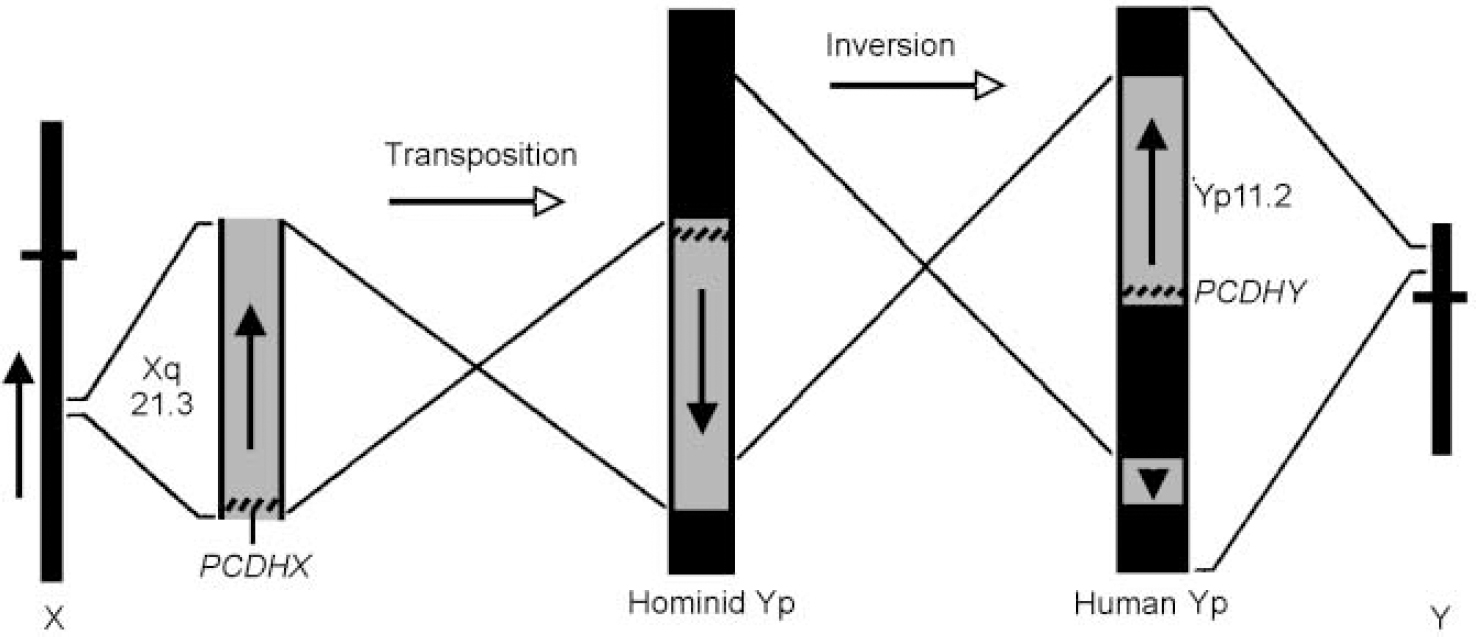
Fig. 3 The transposition from Xq21.3 and subsequent paracentric inversion on Yp, that generated the Xq21.3/Yp11 block of homology and its orientation in modern Homo sapiens. Vertical arrows indicate the orientation of the gene sequence. Yp, Y chromosome short arm; cross-bars on the X and Y chromosome icons indicate centromeres. PCDHX, protocadherinX; PCDHY, protocadherinY. (Adapted from Reference Schwartz, Chan and BrownSchwartz et al, 1998.)
Gene sequences within this block are present on the X and Y chromosomes in humans but only on the X in other primates. Within this region of homology a gene — protocadherinXY — has recently been described (Reference Blanco, Sargent and BoucherBlanco et al, 2000) that is a member of a class of cell adhesion molecules expressed in the brain that have a role in axonal guidance. It is therefore a candidate for H. sapiens-specific characteristics such as cerebral asymmetry (Reference Crow and WrayCrow, 2001). In that there are sequence differences between the X and Y copies, this gene can account for gender differences such as those observed in age of onset of psychosis, lateralisation and the development of verbal ability. According to the X—Y theory of cerebral asymmetry, the chromosomal re-arrangements that led to protocadherinX being represented on the Y as well as the X chromosome were speciation events in hominid evolution (Reference CrowCrow, 2000).
EPIGENETICS OF ASYMMETRY
The particular interest of an X—Y homologous gene subject to recent evolutionary change is its status with respect to X inactivation — the epigenetic process by which the expression of most genes on one X chromosome in females is inhibited (Lyon, Reference Lyon1974, Reference Lyon1999). Genes with a copy on the Y chromosome are protected from this process, although the mechanism of this protection is obscure. It presumably applies to protocadherinXY. Genes that have recently translocated from the X to the Y are in a new environment; the Y copy escapes from X inactivation and there must be a process whereby the state of inactivation of the gene on the X chromosome changes (see Reference Jegalian and PageJegalian & Page, 1998). One possibility is that the protected sequences are those that are able to pair in male meiosis with similar sequences on the Y (Reference BurgoyneBurgoyne, 1982; Reference CrowCrow, 1991; but also see Reference Burgoyne and McLarenBurgoyne & McLaren, 1985). Determining the mechanism and rules that govern this process could be a necessary prelude to an understanding of the variability that is intrinsic to the species.
CONCLUSIONS
ProtocadherinXY in the Xq21.3/Yp region of homology is a candidate determinant of cerebral asymmetry and therefore of the human capacity for language. If the X—Y hypothesis is correct, a component of the variation with respect to this recent and species-specific evolutionary development is epigenetic rather than sequence-based, and it is this, rather than unidentified environmental factors, that accounts for variability of transmission of handedness and psychosis.






eLetters
No eLetters have been published for this article.