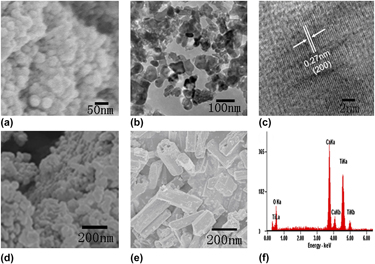
Cover: FIG. 2(c). TEM image of the Janus composite particles of TiO2–poly (VBC–DVB). [Q. Chen, L. Zheng, B. Chen, J. He, H. Huang, and J. Lin: Highly efficient phase transfer catalyst supported on Janus composite particles: synthesis, characterization, and applications. p. 1231].
Articles
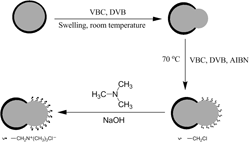
Highly efficient phase transfer catalyst supported on Janus composite particles: Synthesis, characterization, and applications
-
- Published online by Cambridge University Press:
- 20 June 2014, pp. 1231-1236
-
- Article
- Export citation
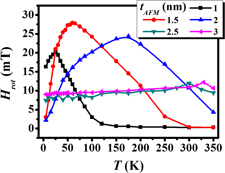
Temperature dependence of static and dynamic magnetic properties in NiFe/IrMn bilayer system
-
- Published online by Cambridge University Press:
- 10 June 2014, pp. 1237-1247
-
- Article
- Export citation
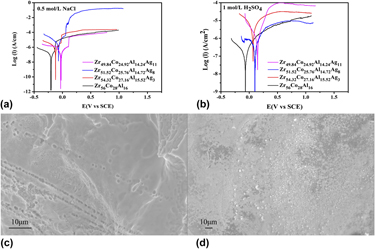
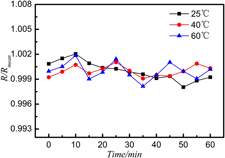
The corrosion and oxidation behavior of Zr-based metallic glasses
-
- Published online by Cambridge University Press:
- 28 May 2014, pp. 1248-1255
-
- Article
- Export citation
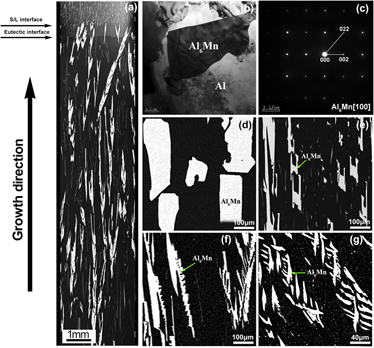
Faceted–nonfaceted growth transition and 3-D morphological evolution of primary Al6Mn microcrystals in directionally solidified Al–3 at.% Mn alloy
-
- Published online by Cambridge University Press:
- 09 June 2014, pp. 1256-1263
-
- Article
- Export citation
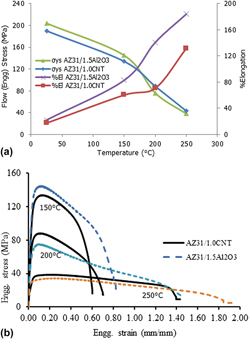
Study of comparative effectiveness of thermally stable nanoparticles on high temperature deformability of wrought AZ31 alloy
-
- Published online by Cambridge University Press:
- 03 June 2014, pp. 1264-1269
-
- Article
- Export citation
Modification of near-eutectic Al–Si alloys with rare earth element samarium
-
- Published online by Cambridge University Press:
- 09 June 2014, pp. 1270-1277
-
- Article
- Export citation
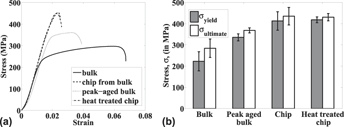
Tensile testing of Al6061-T6 microspecimens with ultrafine grained structure derived from machining-based SPD process
-
- Published online by Cambridge University Press:
- 25 June 2014, pp. 1278-1287
-
- Article
- Export citation
Temperature-dependent electrical properties of graphene nanoplatelets film dropped on flexible substrates
-
- Published online by Cambridge University Press:
- 09 June 2014, pp. 1288-1294
-
- Article
- Export citation
Photocatalytic activity of MTiO3 (M = Ca, Ni, and Zn) nanocrystals for water decomposition to hydrogen
-
- Published online by Cambridge University Press:
- 04 June 2014, pp. 1295-1301
-
- Article
- Export citation
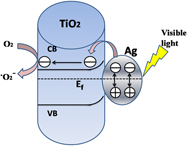
Silver-decorated titanium dioxide nanotube arrays with improved photocatalytic activity for visible light irradiation
-
- Published online by Cambridge University Press:
- 17 June 2014, pp. 1302-1308
-
- Article
- Export citation
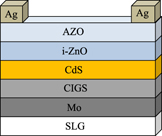
Optimization of solution-processed Cu(In,Ga)S2 by tuning series and shunt resistance
-
- Published online by Cambridge University Press:
- 09 June 2014, pp. 1309-1316
-
- Article
- Export citation
Front Cover (OFC, IFC) and matter
JMR volume 29 issue 11 Cover and Front matter
-
- Published online by Cambridge University Press:
- 25 June 2014, pp. f1-f4
-
- Article
-
- You have access
- Export citation
Back Cover (OBC, IBC) and matter
JMR volume 29 issue 11 Cover and Back matter
-
- Published online by Cambridge University Press:
- 25 June 2014, pp. b1-b2
-
- Article
-
- You have access
- Export citation