Article contents
The corrosion and oxidation behavior of Zr-based metallic glasses
Published online by Cambridge University Press: 28 May 2014
Abstract
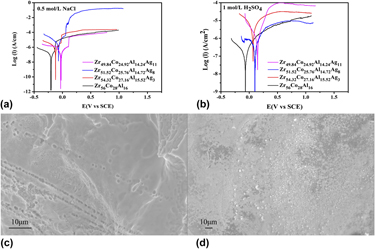
Zr-based bulk metallic glasses are promising engineering materials due to their good glass-forming abilities and unique combination of good strength (∼1.9 GPa) and medium stiffness. In this study, the corrosion behaviors of Zr–Co–Al–Ag BMGs, with silver content from 0 to 11 at.%, in NaCl and H2SO4 solutions were investigated. The corrosion resistance increased when the silver content increased. The oxidation behaviors of Zr–Co–Al–Ag BMGs were also studied. The oxidation kinetics of all the samples obeyed a two-stage parabolic rate law, which consisted of an initial transient oxidation followed by a steady-state oxidation stage. The addition of Ag was found to reduce the oxidation resistance of Zr–Co–Al–Ag BMGs. Some white nodules, possibly cobalt oxide, were observed when the Zr–Co–Al–Ag BMG with 8 at.% Ag was oxidized at high temperature.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 3
- Cited by


