In recent years, the incidence of paediatric non-alcoholic fatty liver disease (NAFLD) has increased worldwide in parallel with childhood obesity rates and, therefore, constitutes an emerging global health concern( Reference Than and Newsome 1 ). NAFLD is one of the most common causes of liver disease among children and adolescents( Reference Yang, Gong and Ye 2 ) and consists of the accumulation of lipids within the hepatocytes in the absence of excess alcohol consumption( Reference Abenavoli, Milic and Peta 3 ). This pathological disorder is strongly associated with insulin resistance and dyslipidaemia and, therefore, it is considered to be the hepatic manifestation of the metabolic syndrome( Reference Ferolla, Silva and De Lourdes 4 , Reference Alkhater 5 ).
Diet and other lifestyle determinants seem to modulate hepatic steatosis development and progression( Reference Zivkovic, German and Sanyal 6 ). For instance, western dietary habits( Reference Oddy, Herbison and Jacoby 7 ) in both normal weight and overweight/obese adolescents, as well as energy intake in children( Reference Anderson, Howe and Fraser 8 ) or dietary sugar intake such as fructose or sucrose in adults( Reference Azaïs-Braesco, Sluik and Maillot 9 ), may have an influence on hepatic fat content and other cardiometabolic risk factors( Reference Ma, Fox and Jacques 10 , Reference Te Morenga, Howatson and Jones 11 ).
Sugar-sweetened beverages (SSB) are the main source of dietary added sugars among children and adolescents, representing around 10–15 % of their dietary energy intake( Reference Keller and Bucher Della Torre 12 , Reference Hu and Malik 13 ). Along with the increase in childhood obesity and hepatic steatosis, SSB consumption has also risen in the last years( Reference Keller and Bucher Della Torre 12 ). In this context, a systematic review and meta-analysis reported higher risk of NAFLD assessed by abdominal ultrasound or liver biopsy in patients consuming SSB( Reference Wijarnpreecha, Thongprayoon and Edmonds 14 ). Other studies have also shown association of SSB consumption with adiposity, type 2 diabetes, the metabolic syndrome and CVD risk factors( Reference Jensen, Nielsen and Husby 15 ).
Dairy desserts and substitutes (DDS) are other common dietary sugar sources among children since they are often flavoured and sugar sweetened( Reference Azaïs-Braesco, Sluik and Maillot 9 , Reference Stern, Piernas and Barquera 16 ). Nevertheless, the sugar added in DDS seems not to play such a detrimental role in cardiometabolic health in comparison with sugar from SSB( Reference Khan and Sievenpiper 17 ). In contrast, the intake of other food groups and dietary components such as fruits and vegetables( Reference Cook, O’Reilly and Goran 18 ), dairy products( Reference Shin, Yoon and Lee 19 ) and fibre( Reference Cantero, Abete and Monreal 20 , Reference Davis, Alexander and Ventura 21 ) may have a protective role against the metabolic syndrome and hepatic fat accumulation. However, the majority of the studies examining the association of dietary intake with hepatic steatosis are conducted in adolescents( Reference Oddy, Herbison and Jacoby 7 , Reference Cook, O’Reilly and Goran 18 ) and adult populations( Reference Jia, Xia and Zhang 22 ). Hence, there are few studies examining the relationship between dietary habits and liver health( Reference Papandreou, Karabouta and Pantoleon 23 , Reference Félix, Costenaro and Gottschall 24 ) or insulin resistance( Reference Visuthranukul, Sirimongkol and Prachansuwan 25 ) in overweight and obese children, and, to the best of our knowledge, none of these studies measured hepatic fat content by MRI, which is considered to be the gold standard.
We hypothesised that the intake of products rich in added sugars, such as SSB consumption, would be associated with higher hepatic fat accumulation, whereas no such association would be observed between DDS and hepatic fat content. Likewise, fibre or fibre-rich foods would be beneficial in the prevention of hepatic steatosis and insulin resistance.
Therefore, the aims of the present study were to examine (i) the association of the consumption of dietary foods and components on hepatic fat content and insulin resistance (homeostatic model assessment for insulin resistance (HOMA-IR)) and (ii) the influence of SSB and DDS, as well as their sugar content, on hepatic fat content and insulin resistance in overweight/obese children.
Methods
Study participants and design
The Prevention of Diabetes in Kids (PREDIKID) study is a randomised controlled trial (ClinicalTrials.gov ID: NCT03027726) examining the effect of a family-based intervention program( Reference Arenaza, Medrano and Amasene 26 ) on diabetes risk of overweight/obese children. The study protocol was approved by the Ethic Committee of Clinical Investigation of Euskadi (PI2014045) and the research was conducted according to the Declaration of Helsinki. Participants were recruited from the Pediatric Endocrinology Unit of the University Hospital of Araba and Primary Care Clinics from Vitoria-Gasteiz and surroundings (North of Spain) in 2017. The main outcome of the trial was insulin resistance( Reference Arenaza, Medrano and Amasene 26 ), whereas the primary outcomes for this sub-study were hepatic fat content and insulin resistance. The inclusion criteria were (1) to be aged between 8 and 12 years, (2) to have overweight or obesity and (3) to meet the international criteria for classification of type 2 diabetes risk. All participants’ parents or legal guardians signed an informed written consent for their children in order to be enrolled in the study. The present cross-sectional study includes baseline data of 110 children (10·6 (sd 1·1) years old, 53·1 % girls) with overweight or obesity participating in the PREDIKID study who had dietary intake and hepatic fat content data.
Anthropometry
Height (SECA 220) and body weight (SECA 760) were measured barefoot according to standard protocols, and waist circumference was measured with a non-elastic tape (SECA 201) following international recommendations. BMI was calculated as body weight divided by height squared (kg/m2) and, thereafter, children were classified as having overweight or obesity according to World Obesity Federation criteria(27). All the anthropometric measurements were carried out by the same trained researcher to avoid interpersonal variability bias. Pubertal stage was directly examined by a paediatrician and classified according to Tanner & Whitehouse( Reference Tanner and Whitehouse 28 ) criteria.
Body composition
Dual-energy X-ray absorptiometry (QDR 4500W; Hologic) was used to evaluate total and abdominal adiposity. Abdominal adiposity was measured by determining three abdominal sections as described elsewhere( Reference Labayen, Ruiz and Vicente-Rodríguez 29 ).
Hepatic fat
Hepatic fat content was measured by MRI (MAGNETOM Avanto; Siemens Healthcare) with phased-array surface coil and a spine array coil equipment. This was provided by the work-in-progress software package by Siemens Medical System (version syngo.MR B17A). Briefly, two different three-dimensional gradient-echo sequences in breath-hold and six-echo acquisition with advanced signal analysis were used for hepatic fat quantification and estimation, respectively, as reported elsewhere( Reference Medrano, Maiz and Maldonado-Martin 30 ). According to the literature, liver proton density fat fraction estimated by MRI correlates well with histologic steatosis grade in children( Reference Schwimmer, Middleton and Behling 31 ), and therefore MRI is considered a non-invasive and accurate method to detect hepatic steatosis in paediatric population( Reference Hsu and Murray 32 ).
In accordance with previous studies, the cut-off value of hepatic fat content established for categorising children with and without hepatic steatosis was 5·5 %( Reference Younossi, Loomba and Rinella 33 , Reference Browning, Szczepaniak and Dobbins 34 ). Therefore, children with a hepatic fat ≥5·5 % were considered as having hepatic steatosis, whereas children with <5·5 % hepatic fat accumulation as not having hepatic steatosis.
Insulin resistance
Insulin resistance was determined by calculating HOMA-IR using fasting blood glucose and insulin values with the following formula: (insulin (µU/ml×glucose (mmol/l))/22·5)( Reference Matthews, Hosker and Rudenski 35 ). Blood sample collection details have been published elsewhere( Reference Medrano, Maiz and Maldonado-Martin 30 ).
Dietary intake assessment
Dietary intake was evaluated, by trained nutritionists, as the mean of two non-consecutive 24 h-recalls during a time span of a week. Children reported, with help of their parents, all the foods and beverages consumed the day before the interview. Pictures of food servings were used to help the participants to estimate food intake. Nutritional composition data was obtained by EasyDiet software. Thereby, energy intake and macro- and micro-nutritional composition data from both interviewed days was obtained. Regarding the food groups, each dietary recall was examined thoroughly, and thereafter, the food consumed was classified into the different food groups so as to obtain the daily food-group intake. Afterwards, the mean consumption of the dietary components consumed in both interviewed days was computed.
Fruits and vegetables were analysed together. Detailed information about foods included in each food category and explanation about each term is available in online Supplementary Table S1. Participants were asked to report product brands, particularly for SSB and DDS, to obtain detailed nutritional composition data, that is, sugar and energy content of each food and beverage. SSB consumption included SSB, while low- or non-energetic beverages were excluded due to the reduction of sugar and replacement for artificial non-energetic sweeteners. DDS were considered milk-derived products with added sugar when its sugar content was ≥6 g/100 g product. The current sugar cut-off value was established based on the natural lactose content of dairy products, assuming that dairy products with higher sugar content may have sugar added in their composition. Also, all dairy product substitutes such as soya-, oat- or almond-beverages were included in DDS, but no cut-off value for sugar content was used since they are not milk-based.
Furthermore, total added sugar was calculated by removing the sugar amount in natural sugar sources such as fruits and dairy products from total dietary sugar intake.
Physical activity
Accelerometry was used as an objective measurement of total physical activity (wActisleep-BT and wGT3X-BT; ActiGraph). All participants had to wear an accelerometer on the non-dominant wrist during a week. Euclidean Norm Minus One was used to quantify the acceleration related to the movement registered expressed in mg using R software (version 3.1.2, www.cran.r-project.org) with the GGIR package (version 1.5-12, https://cran.r-project.org/web/packages/GGIR/).
Statistical analysis
As the variables did not show a normal distribution, non-parametrical Mann–Whitney U tests were used to examine the differences in continuous variables (i.e. biological characteristics and dietary intake) between children with and without hepatic steatosis, whereas the χ 2 test was used for categorical variables. Linear regression analyses were used to examine the association between dietary components (independent variables) and hepatic fat content and insulin resistance (dependent variables). Potential confounders such as sex, age, energy intake and maternal educational level (model 1) and additionally body fat percentage (model 2) or abdominal adiposity (model 3) and dietary simple sugar intake (model 4) were used as covariates in the analyses. Variables with a non-normal distribution were logarithmically transformed for linear regression analyses. Sensitivity analyses were performed in dietary variables and hepatic fat content analysis adjusting additionally for HOMA-IR, physical activity and parental BMI. Collinearity diagnosis tests were also performed between dietary factors to examine whether they were related to one another. Statistical analyses were carried out with the statistical software SPSS version 20.0 (SPSS Inc.) with the significance level of α=0·05.
Results
Biological characteristics and dietary intake of participants with and without hepatic steatosis are shown in Table 1. The proportion of children with hepatic steatosis was 36·4 %. Children with hepatic steatosis had significantly higher BMI z-score (P<0·03), waist circumference (P<0·01), total and abdominal adiposity (P<0·01), hepatic fat content (P<0·001) and HOMA-IR (P<0·01) compared to children without hepatic steatosis. Likewise, the percentage of children with obesity was higher in the group with hepatic steatosis (P<0·03), while age, sex, high maternal educational level, parental obesity and diabetes and total physical activity were similar between both groups (P>0·05), non-Caucasian ethnicity was higher among children with hepatic steatosis (P<0·03). In contrast, no significant differences were observed in the intake of macronutrients, simple sugars, added sugars, fibre, cereals, fruits and vegetables, legumes, nuts, dairy products, fish and shellfish and meat and meats products between children with and without hepatic steatosis (P>0·1). The consumption of SSB and the sugar content of SSB tended to be higher in children with hepatic steatosis than in their peers without hepatic steatosis (P<0·1).
Table 1 Biological characteristics and dietary intake of participants with and without hepatic steatosis of the children participating in the Prevention of Diabetes in Kids (PREDIKID) study (Mean values and standard deviations)
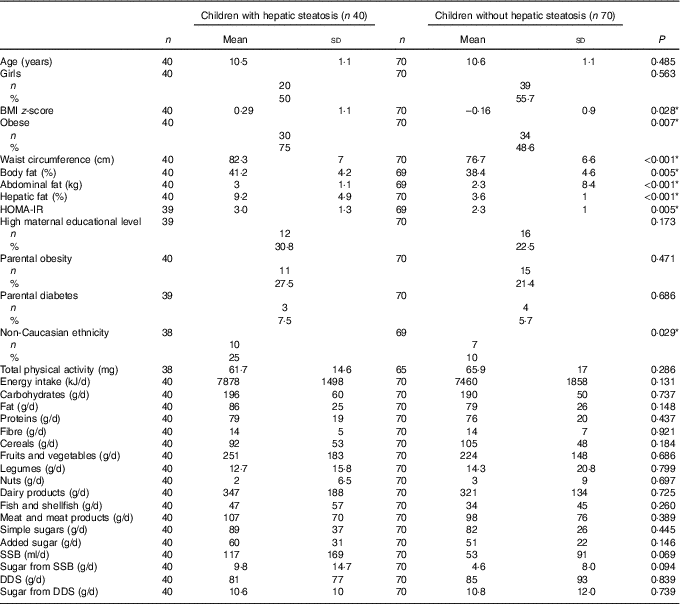
HOMA-IR, homeostatic model assessment for insulin resistance; SSB, sugar-sweetened beverages; DDS, dairy desserts and substitutes.
* P <0.05.
Table 2 shows the associations of dietary components with hepatic fat content and insulin resistance. A negative association was observed between cereal intake and hepatic fat content regardless of sex, age, energy intake and maternal educational level (P<0·02, model 1). This association was diminished, but remained significant, when both total (model 2) and abdominal fat (model 3) were entered into the model (P<0·05). No statistically significant associations were found between the rest of dietary components and hepatic fat content and insulin resistance in overweight/obese children.
Table 2 Association of dietary energy and macronutrient intake, and other dietary components with hepatic fat content and insulin resistance in overweight/obese childrenFootnote *
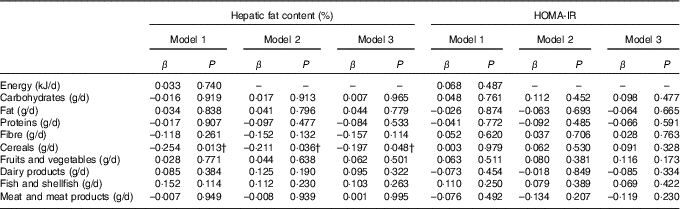
HOMA-IR, homeostatic model assessment for insulin resistance.
* Model 1: adjusted for sex, age, energy intake and maternal educational level; model 2: model 1 additionally adjusted for body fat percentage; model 3: model 2 additionally adjusted for abdominal adiposity.
† P<0·05.
The associations of SSB and DDS intake and the intake of their sugar contents with hepatic fat and HOMA-IR are shown in Table 3. The results showed that both SSB consumption and the intake of sugar from SSB were significantly associated with hepatic fat content regardless of sex, age, energy intake and maternal educational level (P<0·02, model 1). These relationships were still significant when either body fat percentage or abdominal fat were included into the model (P<0·02, models 2 and 3). Interestingly, the association of SSB consumption and the intake of sugar from SSB with hepatic fat content remained statistically significant even after further adjustment for total simple sugar intake (P<0·05, model 4). In a sensitivity (additional adjustment) analysis with insulin resistance (β=0·212, P=0·036 and β=0·204, P=0·045 for SSB and sugar from SSB, respectively), physical activity (β=0·217, P=0·032 and β=0·206, P=0·043 for SSB and sugar from SSB, respectively) and parental BMI (β=0·207, P=0·037 and β=0·199, P=0·047 for SSB and sugar from SSB, respectively) this association remained statistically significant. Continuous logistic regression was performed to analyse the effect of SSB consumption in children with and without hepatic steatosis (sex, age, maternal educational level and energy intake adjustments); and even though it was not statistically significant, a trend to signification was observed (OR 1·003, 95 % CI 1·000, 1·007, P=0·051). On the contrary, no significant relationships were observed between DDS consumption and sugar from DDS with hepatic fat content in overweight/obese children (P>0·05). Neither SSB nor DDS consumption were associated with insulin resistance (P>0·05). No collinearity was found between dietary factors.
Table 3 Associations of sugar-sweetened beverages (SSB) and dairy desserts and substitutes (DDS) consumption as well as their sugar content with hepatic fat content and insulin resistance (homeostatic model assessment for insulin resistance (HOMA-IR)) in overweight/obese childrenFootnote *
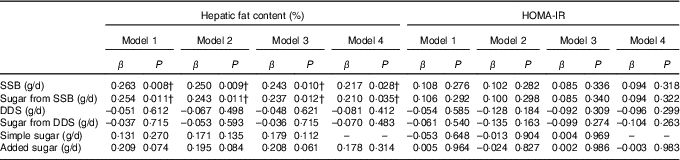
* Model 1: adjusted for sex, age, energy intake and maternal education level; model 2: model 1 additionally adjusted for body fat percentage; model 3: model 2 additionally adjusted for abdominal adiposity; model 4: model 3 additionally adjusted for simple sugar intake.
† P<0.05.
Discussion
The present study examined the influence of several food groups and dietary components on hepatic fat content and insulin resistance among pre-adolescent overweight/obese children. The main findings were that cereal intake was negatively associated with hepatic fat content but not with insulin resistance, whereas the rest of the food groups and macronutrients were associated neither with hepatic fat content nor with insulin resistance. Moreover, SSB consumption and sugar from SSB were positively associated with hepatic fat accumulation, but not with insulin resistance. In contrast, we did not observe any significant relationships between DDS and hepatic fat content or insulin resistance among overweight/obese children aged 8–12 years.
Almost half of the children in the study (36·4 % of the participants) presented with hepatic steatosis. It is well-known that excess adiposity increases the risk of having NAFLD( Reference Barshop, Sirlin and Schwimmer 36 ). Our findings about prevalence are in agreement with those from two previous studies. One of them reported that whilst the prevalence of paediatric hepatic steatosis is lower than 10 % in general population, the prevalence among children with obesity can reach 80 %( Reference Alisi, Feldstein and Villani 37 ). According to other study, the prevalence of NAFLD in children with normal weight was 2·6 %, which increased to 20–77 % among obese children and adolescents ( Reference Reinehr, Schmidt and Toschke 38 ). Nevertheless, it should be pointed out that the range of prevalence might vary depending on the diagnosis method used for measuring hepatic fat content. In this study, hepatic fat content was measured by MRI, which is a more accurate method compared to ultrasound, and in fact, it has been considered a non-invasive appropriate alternative for paediatric population( Reference Younossi, Loomba and Rinella 33 ). Furthermore, and in line with other studies( Reference Browning, Szczepaniak and Dobbins 34 ), ethnic differences between Hispanic, Black and White population were observed in the prevalence of hepatic steatosis. Regarding body composition, children with hepatic steatosis had higher values of waist circumference as well as total and abdominal adiposity compared to children without hepatic steatosis. On the contrary, participants with hepatic steatosis did not show higher values of BMI, which demonstrates the limitations of using BMI as a proxy of body fatness in children( Reference Freedman and Sherry 39 ). Accordingly, based on the strong association between abdominal adiposity and hepatic steatosis, Alisi et al. ( Reference Alisi, Manco and Vania 40 ) reported that abdominal obesity is more powerful than BMI in predicting hepatic steatosis.
Overall, we found only few differences in dietary intake variables between children with and without hepatic steatosis. In contrast to our results, a previous study reported that adolescents with hepatic steatosis had higher intake of total fat and fried foods compared to their peers without hepatic steatosis( Reference Macintosh, Hay and Wicklow 41 ). However, in the present study children with hepatic steatosis tended to consume more SSB compared to children without hepatic steatosis, which is consistent with the association between SSB consumption and NAFLD( Reference Ma, Fox and Jacques 10 ). Our observations suggest that cereal intake may be protective against hepatic steatosis development. On the contrary, Georgoulis et al. ( Reference Georgoulis, Kontogianni and Tileli 42 ) reported that refined grains were associated with higher likelihood of having NAFLD in adults, whereas whole grain consumption favourably affected clinical characteristics of participants with NAFLD as a result of being rich in fibre and having lower glycaemic index. Unfortunately, due to the infrequent intake of whole grains by children from the PREDIKID study, we could not examine the influence of whole cereals on hepatic health. On the other hand, in disagreement with other studies in which whole cereal intake was inversely associated with insulin resistance among adolescents( Reference Steffen, Jacobs and Murtaugh 43 ), no association was found between cereal intake and HOMA-IR in overweight/obese children.
We observed that 39·8 and 65·5 % of the children consumed SSB and DDS, respectively (data not shown). Moreover, we found a positive association between SSB consumption and sugar in SSB and hepatic fat content regardless of sex, age, energy intake, maternal educational level, body fat percentage, abdominal obesity and total simple sugar intake. Of note, while in the USA SSB are primarily sweetened with high fructose maize syrup, in Europe sucrose is commonly used as the main sweetener( Reference Malik and Hu 44 ), which is a disaccharide composed of fructose and glucose. Although several studies report the association of both fructose( Reference Alwahsh and Gebhardt 45 ) and SSB consumption( Reference Wang, Light and Loughlin 46 , Reference Chan, Lin and Huang 47 ) with adiposity and cardiometabolic risk factors in adults and adolescents, few studies focused on children to date. Furthermore, to the best of our knowledge, this is the first study examining the influence of SSB consumption on hepatic fat content in overweight/obese children. Interestingly, neither SSB nor sugar from SSB was associated with insulin resistance. In addition, based on the fact that impaired insulin signalling originated by dysfunctional adipose tissue induces insulin resistance, insulin resistance might be a secondary effect of excess hepatic fat. Despite the lack of the association in our study, other authors reported that daily intake of SSB was associated with increased HOMA-IR in adolescents( Reference Kondaki, Grammatikaki and Jime 48 ). The possible mechanism of how fructose can induce NAFLD has largely been studied in animal models. Briefly, fructose is absorbed from the intestine into the portal vein and goes directly to the liver, where it stimulates de novo lipogenesis by promoting hepatic lipid accumulation( Reference Alisi, Manco and Vania 40 , Reference Wang, Light and Loughlin 46 ), which may explain NAFLD development. Nonetheless, not only fructose but also sucrose-containing beverages have been shown to increase hepatic fat as well as visceral and muscle fat and TAG( Reference Maersk, Belza and Stodkilde-jorgensen 49 ).
In contrast, DDS consumption was not significantly associated with hepatic fat content and insulin resistance in our sample of overweight/obese children. This finding is in line with previous studies suggesting that dairy fat intake as well as other nutrients like Ca (which are mostly present in DDS, but not in SSB) could be protective against hepatic fat accumulation in adults( Reference Kratz, Marcovina and Nelson 50 ) and abdominal adiposity in female adolescents( Reference Castro Burbano and Fajardo Vanegas 51 ). In addition, the fact that DDS are commonly consumed with a meal, whereas people tend to drink SSB between meals, could also explain the influence on these outcomes. Accordingly, previous studies reported that snacking promotes liver fat accumulation( Reference Piernas and Popkin 52 , Reference Koopman, Caan and Nederveen 53 ). As a consequence of the rise of SSB consumption in children, initiatives such as the implementation of SSB-associated tax in several countries have been carried out to prevent obesity and related comorbidities. The purchases of SSB decreased after Mexico implemented a tax, so that this seems to be a successful intervention from global policies( Reference Colchero, Molina and Guerrero-l 54 ).
Strengths and limitations
The use of MRI to measure hepatic fat content should be considered as study strength for its accuracy, while ultrasonography is used in most of the other studies. Moreover, detailed nutritional composition information of SSB and DDS was obtained by analysing the labels of products brands. However, the current study also has some limitations. These findings should be analysed carefully due to its cross-sectional design and, therefore, prospective studies are needed since experimental evidence would strengthen these cross-section observations.
Conclusions
The main findings in the present study suggest that cereal intake seems to be associated with lower liver fat content, whereas SSB consumption and sugar from SSB might increase hepatic fat content among overweight/obese children. In contrast, neither DDS consumption nor sugar from DDS seems to modulate cardiometabolic risk factors, and last but not least, no association was found between dietary intake and insulin resistance in children with excess adiposity. Our results suggest that healthy lifestyle intervention programs are needed to improve dietary habits as well as to increase the awareness of the detrimental effects of SSB consumption early in life.
Acknowledgements
The authors acknowledge all the children and their parents or legal guardians for participating in the study. The authors also thank the paediatricians for their collaboration in recruiting participants and to the pre- and post-graduate students for their collaboration in both data collection and intervention program sessions.
This project was supported by the Spanish Ministry of Industry and Competitiveness (DEP2016-78377-R), by ‘Fondos Estructurales de la Unión Europea (FEDER), Una manera de hacer Europa’ and by the University of the Basque Country (GIU14/21). This work was also supported by grants from Spanish Ministry of Economy and Competitiveness (RYC 2010-05957; RYC-2011-09011), by the Education Department of the Government of the Basque Country (PRE_2016_1_0057, PRE_2017_2_0224 and PRE_2018_2_0057), by the Spanish Ministry of Education, Culture and Sports (FPU14/03329), and by the Swedish Society of Medicine.
I. L. conceived and designed the study, L. A. and I. L. drafted the manuscript, L. A., M. M., M. O. and I. L. collected the data and L. A., M. M., M. O., I. H., I. D., H. H. and I. L. were involved in the interpretation of the results. All the authors critically revised the manuscript for important intellectual content and approved the final version of the submitted manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519000436






