I read with great interest the recent article by Kishimoto et al. (Reference Kishimoto, Chawla, Hagi, Zarate, Kane, Bauer and Correll2016) about meta-analysis of the antidepressant effects of the N-methyl-d-aspartate receptor (NMDA-R) antagonist ketamine (racemate) and non-ketamine NMDA-R antagonists (traxoprodil, lanicemine, rapastinel) in patients with major depressive disorder (MDD) and bipolar disorder. The authors examined the time trajectory of efficacy of ketamine and non-ketamine NMDA-R antagonists. The greater reduction in depressive symptoms by ketamine infusion started as early as within 40–60 min, peaking on day 1, and lasting until days 5–8, with maintenance of superior remission and response status until days 3–5 and 7, respectively. Effect sizes of ketamine ranged from medium to high (−0.38 to −1.00). In contrast to ketamine, single infusion of non-ketamine NMDA-R antagonists was only significantly superior to placebo at one assessment time point (days 5–8) with a small to medium effect size (−0.37). Together, it is likely that non-ketamine NMDA-R antagonists have smaller effect sizes than ketamine, although the reason underlying this difference remains unclear.
Ketamine (or RS-ketamine) (K i = 0.53 µ m for NMDA-R) is a racemic mixture containing equal parts of R-ketamine and S-ketamine (esketamine). Esketamine shows an approximately 3- to 4-fold greater anaesthetic potency and greater undesirable psychotomimetic side effects, compared with R-ketamine (Domino, Reference Domino2010). This is related to the fact that esketamine (K i = 0.30 µ m) has an approximately 4-fold greater affinity for NMDA-R relative to R-ketamine (K i = 1.4 µ m) (Fig. 1) (Ebert et al. Reference Ebert, Mikkelsen, Thorkildsen and Borgbjerg1997). We reported that R-ketamine showed greater potency and longer-lasting antidepressant effects than esketamine in animal models of depression (Zhang et al. Reference Zhang, Li and Hashimoto2014; Yang et al. Reference Yang, Shirayama, Zhang, Ren, Yao, Ma, Dong and Hashimoto2015). It is therefore unlikely that NMDA-R has a major role in the long-lasting antidepressant effects of R-ketamine, although antagonism at this receptor may promote its rapid antidepressant action (Hashimoto, Reference Hashimoto2014; Zhang et al. Reference Zhang, Li and Hashimoto2014; Yang et al. Reference Yang, Shirayama, Zhang, Ren, Yao, Ma, Dong and Hashimoto2015). Unlike esketamine, R-ketamine does not induce psychotomimetic side effects and abuse potential in rodents (Yang & Hashimoto, Reference Yang and Hashimoto2014; Yang et al. Reference Yang, Shirayama, Zhang, Ren, Yao, Ma, Dong and Hashimoto2015). Furthermore, a single dose of esketamine, but not R-ketamine, resulted in loss of parvalbumin (PV)-immunoreactivity in the prefrontal cortex (Yang et al. Reference Yang, Shirayama, Zhang, Ren, Yao, Ma, Dong and Hashimoto2015). A recent study using [11C]raclopride and positron emission tomography showed a marked reduction of dopamine D2/3 receptor binding in the conscious monkey striatum after single infusion of esketamine, but not R-ketamine (Hashimoto et al. Reference Hashimoto, Kakiuchi, Ohba, Nishiyama and Tsukada2016). Singh et al. (Reference Singh, Fedgchin, Daly, Xi, Melman, De Bruecker, Tadic, Sienaert, Wiegand, Manji, Drevets and Van Nueten2015) reported that psychotomimetic side effects were the highest at 40 min after single infusion of esketamine although a rapid-onset antidepressant effect was shown in treatment-resistant patients with MDD. Considering the role of dopamine release in psychosis, it is likely that marked release of dopamine from presynaptic terminals in the striatum could be associated with psychotomimetic side effects in humans after a single infusion of ketamine or esketamine.
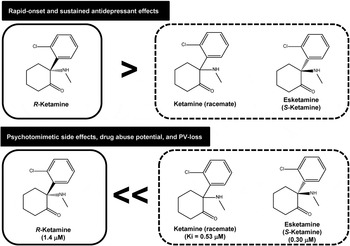
Fig. 1. Antidepressant and side effects of R-ketamine, ketamine (racemate) and esketamine in preclinical models of depression suggest that R-ketamine has greater potency and longer-lasting antidepressant effects than ketamine (racemate) and esketamine (S-ketamine) (Zhang et al. Reference Zhang, Li and Hashimoto2014; Yang et al. Reference Yang, Shirayama, Zhang, Ren, Yao, Ma, Dong and Hashimoto2015). Furthermore, preclinical studies using locomotion, prepulse inhibition, conditioned place preference and parvalbumin (PV)-immunohistochemistry suggest that ketamine and esketamine, but not R-ketamine, could cause psychotomimetic effects, drug abuse potential and PV-loss in the prefrontal cortex (Yang et al. Reference Yang, Shirayama, Zhang, Ren, Yao, Ma, Dong and Hashimoto2015, Reference Yang, Han, Zhang, Ren and Hashimoto2016). A clinical study using esketamine showed marked psychotomimetic side effects in major depressive disorder patients after administration of esketamine (Singh et al. Reference Singh, Fedgchin, Daly, Xi, Melman, De Bruecker, Tadic, Sienaert, Wiegand, Manji, Drevets and Van Nueten2015). Since the psychotomimetic effects of ketamine are known to be associated with N-methyl-d-aspartate receptor (NMDA-R) antagonism, it is likely that the use of R-ketamine is safer than esketamine or ketamine in the treatment of depression. The values in the parentheses are K i values for NMDA-R in the brain (Ebert et al. Reference Ebert, Mikkelsen, Thorkildsen and Borgbjerg1997).
The authors also pointed out that the transient efficacy lasting 1 week post-infusion of ketamine has stimulated multi-infusion studies (Kishimoto et al. Reference Kishimoto, Chawla, Hagi, Zarate, Kane, Bauer and Correll2016). Studies using repeated ketamine (or esketamine) infusions resulted in the marked psychotomimetic side effects after an each infusion although antidepressant effects were shown (Aan het Rot et al. Reference Aan het Rot, Collins, Murrough, Perez, Reich, Charney and Mathew2010; Murrough et al. Reference Murrough, Perez, Pillmer, Stem, Parides, aan het Rot, Collins, Mathew, Charney and Iosifescu2013; Singh et al. Reference Singh, Fedgchin, Daly, Xi, Melman, De Bruecker, Tadic, Sienaert, Wiegand, Manji, Drevets and Van Nueten2015). Interestingly, there was no difference in dissociative, psychotomimetic, or high feeling between responders and non-responders (Murrough et al. Reference Murrough, Perez, Pillmer, Stem, Parides, aan het Rot, Collins, Mathew, Charney and Iosifescu2013), suggesting that ketamine's antidepressant effects are not associated with its psychotomimetic effects. Recently, we reported that repeated, intermittent administration of esketamine, but not R-ketamine, caused loss of PV-immunoreactivity in the prefrontal cortex of mouse brain (Yang et al. Reference Yang, Han, Zhang, Ren and Hashimoto2016). Since loss of PV-immunoreactivity in the prefrontal cortex may be associated with psychosis and γ-oscillation deficits in schizophrenia (Gonzalez-Burgos et al. Reference Gonzalez-Burgos, Cho and Lewis2015), it is possible that repeated administration of esketamine or ketamine may have long-lasting detrimental side effects in the prefrontal cortex of humans.
In conclusion, the use of R-ketamine in the treatment of depression would provide a new therapeutic approach by reducing the detrimental side effects of ketamine.
Acknowledgements
The study was supported by a Grant-in-Aid from the Minister of Education, Culture, Sports, Science, and Technology of Japan (to K.H., no. 24116006).
Declaration of Interest
K.H. is an inventor on a field patent application on ‘The use of R-ketamine in the treatment of psychiatric diseases’ by Chiba University. In addition, K.H. has received research support from Dainippon-Sumitomo, Mochida, Otsuka and Taisho.



