Brief History
Under the umbrella of the Portuguese Healthy Family Study, we designed and conducted seven substudies aimed at increasing our understanding of familial resemblance in body shape and composition, metabolic syndrome, physical activity and physical fitness. These studies had different degrees of complexity in terms of sample sizes, sets of phenotypes and geographical locations. In the first substudy, ‘Genetic and Environmental Factors in Physical Activity Levels of Portuguese Familes’, we sampled 3378 nuclear families (12,385 subjects) from mainland Portugal (Pereira et al., Reference Pereira, Katzmarzyk, Gomes, Elston and Maia2018a; Seabra et al., Reference Seabra, Mendonca, Goring, Thomis and Maia2008) and focused largely on familial resemblance in physical activity phenotypes. The second substudy, ‘Physical Activity and Metabolic Syndrome’ (Campos, Maia, da Silva et al., Reference Campos, Maia, da Silva, Seabra, Lopes, Freitas and Bacalhau2007; Campos, Maia, Seabra et al., Reference Campos, Maia, Seabra, Freitas, da Silva and Lopes2007), was geographically located in the Azores islands and included 133 nuclear families (410 subjects) aiming to best understand familial links between physical activity and metabolic syndrome. Substudy 3, ‘Healthy Camacha: The Study in St. Cruz Families’ (Maia et al., Reference Maia, Freitas and Silva2008), was located in the Madeira islands. Two hundred and ninety subjects (88 families) were assessed on physical activity, body composition, nutritional habits, health perceptions and metabolic syndrome. Substudy 4, ‘Active Vouzela: An Auxological and Epidemiological Study’ (Maia et al., Reference Maia, Seabra and Garganta2009), gathered information on 260 families (802 subjects) to explore familial resemblance in body composition, physical activity, nutritional behaviors, metabolic syndrome and household location. The fifth substudy, ‘Heathy Living in St. Tirso: A Three-year Longitudinal Study’, investigated families across 3 years (year 1, 63 families; year 2, 78 families, year 3, 44 families). More than 1000 children and adolescents were followed for 3 consecutive years, and the phenotypes of interest included body composition, health perceptions, physical activity, nutritional behaviors, metabolic syndrome and bone health. The sixth substudy, ‘Genetic and Environmental Factors in Physical Activity, Nutrition, Health Perceptions, Body Composition, and Metabolic Syndrome’, concentrated its aims on the genetic and environmental architecture of links among physical activity, nutrition, health perceptions, body composition and metabolic syndrome. Finally, the seventh substudy, ‘The Portuguese Sibling Study on Growth, Fitness, Lifestyle and Health’, investigated physical growth, body composition, physical fitness, physical activity, metabolic syndrome and health behaviors in a cohort of siblings (1583 siblings pairs aged 9–20 years).
Recruitment Procedures
Our main recruitment sites were Portuguese public schools located in mainland Portugal, and the Azores and Madeira Islands. Our modus operandi was always the same: first, the principal investigator presented the protocol to the board of directors of each school; second, if accepted, the study aims and methodology were presented to the Physical Education teachers whose help was vital to contact students, mostly in their classes, and from students to their families; third, children and adolescents were invited to participate in the study along with their siblings and parents; fourth, the research team contacted all families interested in participating and was available to respond to all questions parents might like to ask.
All projects were approved by the ethics committee of the Faculty of Sport, University of Porto, and written informed consent was obtained from all subjects including the legal representatives of all youth.
Measures
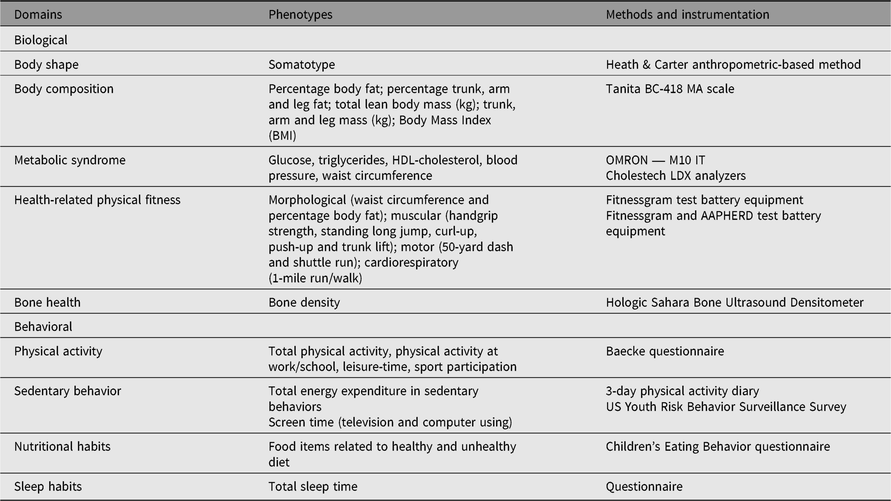
Table 1 depicts research domains, phenotypes and instrumentation used in the various substudies of the Portuguese Healthy Families Study. Research foci were on physical growth, physical fitness, lifestyle and health markers, mirrored in various sets of phenotypes: biological characteristics (body shape, body composition, metabolic syndrome, bone health and health-related physical fitness), and behavioral characteristics (physical activity, sedentary behavior, nutrition and sleep habits). All measurements were made according to standardized protocols, and instrumentation was suited to field research with hundreds or thousands of subjects. Further, all team members were trained by the principal investigator, and when necessary by international co-investigators.
Table 1. Common domains, phenotypes, methods and instrumentation used in the Portuguese Healthy Family Study
Main Findings
A summary of phenotypes, sample, aims, statistical analysis and software used in all studies is presented in Tables 2–5. Sample sizes varied from 107 to 3378 nuclear families (only two generations), that is, from 422 to 12,385 subjects; in siblings, sample sizes also varied from 333 pairs (679 subjects) to 540 pairs (1010 subjects). All published studies to date have used cross-sectional data; we have not yet analyzed data on bone health, nutrition and sleep habits. We relied on top-down genetic epidemiological approaches, and the results comprise measures of resemblance (intraclass correlation and heritability), as well as estimates of gene-by-environment interactions. Given the level of clustering in sibling data, we relied on multilevel models to estimate similarity across sib-types and fitted all models, controlling for the effects of different sets of covariates.
Table 2. Summary of studies examining familial resemblance in body shape and body composition
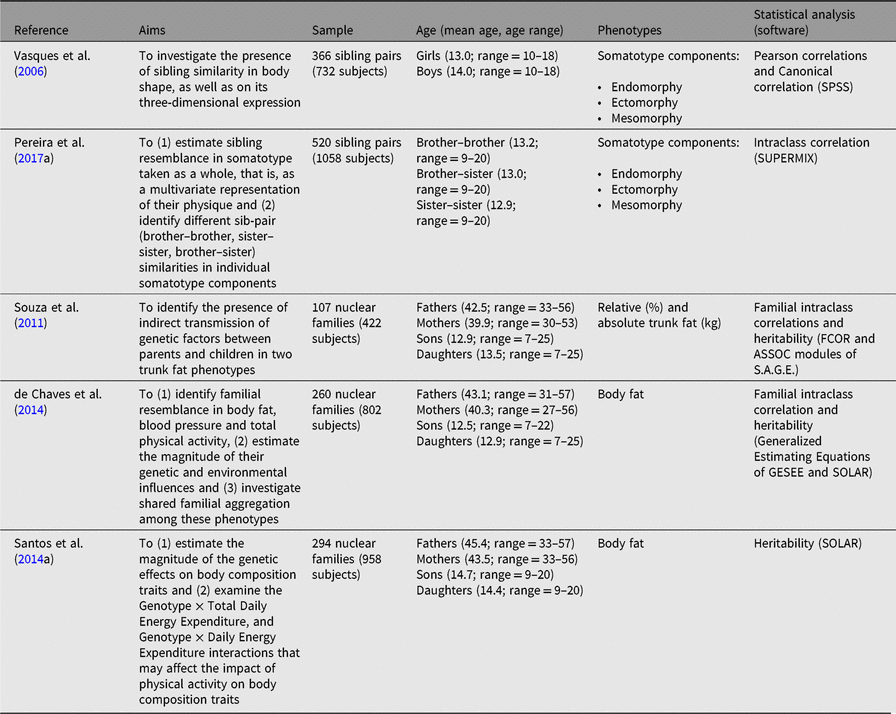
Table 3. Summary of studies examining familial resemblance in metabolic syndrome
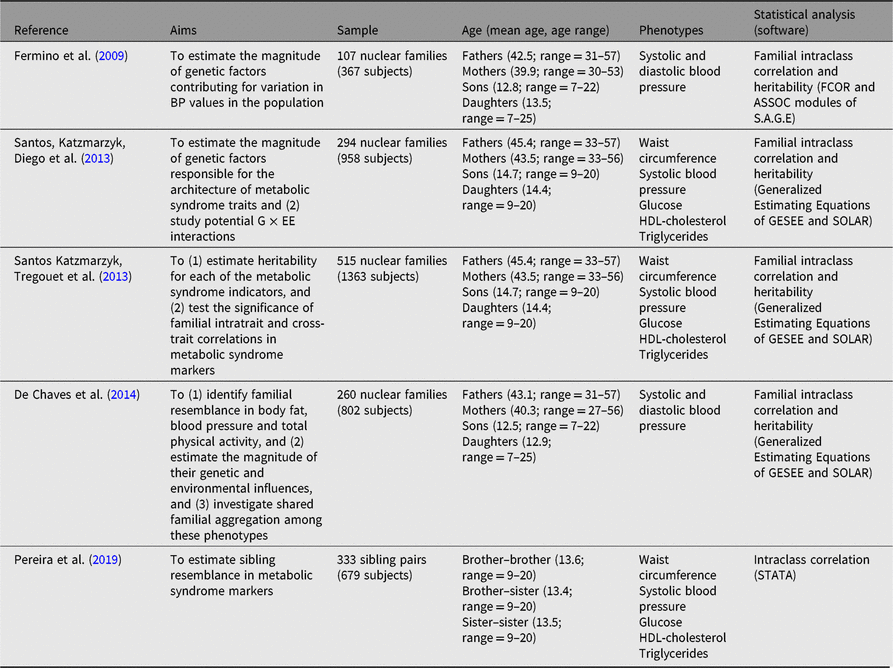
Note: G × EE = genotype by energy expenditure; HDL-cholesterol = high-density lipoprotein cholesterol.
Table 4. Summary of studies examining familial resemblance in physical activity and sedentary behavior
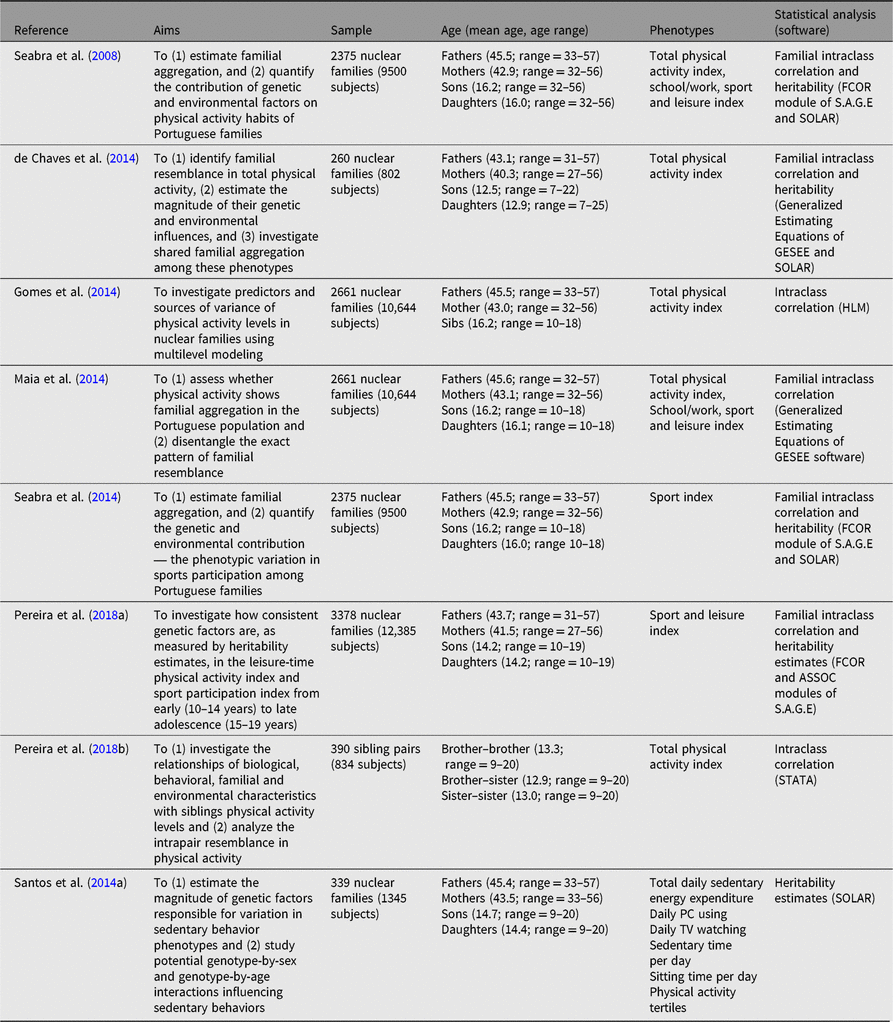
Table 5. Summary studies examining familial resemblance in physical fitness

Body Shape — Somatotype
Body shape, or somatotype, can be defined as the overall representation of an individual’s physique, irrespective of its size, and has historically been rooted in the field of constitutional psychology (Sheldon et al., Reference Sheldon, Stevens and Tucker1940). Somatotype is objectively described in terms of three components based on the Heath–Carter anthropometric method (Heath & Carter, Reference Heath and Carter1967): endomorphy (relative fatness), mesomorphy (musculoskeletal robustness) and ectomorphy (physique linearity). These components are rated on a continuous scale, and from these it is possible to identify the physique dominance and classify individual somatotypes.
Human variation in somatotype has been widely investigated in a variety of settings (for an extensive summary, see Carter & Heath, Reference Carter and Heath1990). It has consistently been related to sports performance (Carter, Reference Carter and Carter1982; Eiben, Reference Eiben1972; Giannopoulos et al., Reference Giannopoulos, Vagenas, Noutsos, Barzouka and Bergeles2017; Ryan-Stewart et al., Reference Ryan-Stewart, Faulkner and Jobson2018; Sterkowicz-Przybycien et al., Reference Sterkowicz-Przybycien, Sterkowicz, Biskup, Zarow, Kryst and Ozimek2019), and it has also been linked to health-related outcomes (Singh, Reference Singh, Bhasin and Bhasin2007) such as coronary heart disease (Williams et al., Reference Williams, Jones, Bell, Davies and Bourne1997), metabolic syndrome (Katzmarzyk et al., Reference Katzmarzyk, Malina, Song and Bouchard1998), blood pressure (Herrera et al., Reference Herrera, Rebato, Hernandez, Hernandez-Valera and Alfonso-Sanchez2004) and osteoporosis (Saitoglu et al., Reference Saitoglu, Ardicoglu, Ozgocmen, Kamanli and Kaya2007).
In our recent research with Portuguese data, two articles dealt with sibling resemblance in somatotype (Table 2). Vasques et al. (Reference Vasques, Lopes, Seabra, Silva and Maia2006) sampled 366 sibling pairs aged 10–18 years from north-east mainland Portugal, whereas Pereira et al. (Reference Pereira, Katzmarzyk, Gomes, Souza, Chaves, Santos and Maia2017a) examined 520 sibling pairs aged 9–20 years from the north and central regions of mainland Portugal. Vasques et al. (Reference Vasques, Lopes, Seabra, Silva and Maia2006) controlled their analysis only for age and sex, while Pereira et al. (Reference Pereira, Katzmarzyk, Gomes, Souza, Chaves, Santos and Maia2017a) controlled for an extensive set of covariates, including age, age2, age3, total physical activity (TPA), age-by-TPA, age2-by-TPA and by age3-by-TPA interactions, as well as socioeconomic status. However, the results are consistent between the two studies, with same-sex siblings being more similar in physique than opposite-sex sibs, regardless of the component under scrutiny. Further, a moderate familial index was reported when somatotype was considered as a gestalt of body physique. These results highlight the key role of familial and genetic factors in regulating the expression of each physique component, as well as the somatotype as a whole. Further, these studies provide interesting contributions to better understand the complexities of shared and nonshared factors in the ways that siblings resemble each other in their physique. Yet, future research could connect, in a multivariate fashion, adolescents’ somatotypes with their health-related physical fitness components, food behaviors, sedentariness and various health outcomes. If these links could be approached longitudinally, they would provide important information for more efficient intervention programs at school or sport clubs settings within their sports for all programs.
Body Composition
Across all of our Portuguese studies, body composition phenotypes were estimated with a reliable and valid instrument (Kabiri et al., Reference Kabiri, Hernandez and Mitchell2015) — a portable bio-electrical impedance scale (TANITA BC-418 MA Segmental Body Composition Analyzer, Tanita Corporation, Japan). Four studies with Portuguese nuclear families were published (Table 3): Fermino et al. (Reference Fermino, Seabra, Garganta, Valdivia and Maia2008) and Souza et al. (Reference Souza, Chaves, Santos, Fermino, Garganta, Seabra and Maia2011) used 107 nuclear families, Santos et al. (Reference Santos, Katzmarzyk, Diego, Gomes, Santos, Blangero and Maia2014b) examined 294 nuclear families and de Chaves et al. (Reference de Chaves, Baxter-Jones, Santos, Gomes, dos Santos, de Souza and Maia2014) used 260 nuclear families. Familial correlations and heritabilities were estimated using S.A.G.E. v5.3 (2005) FCOR and ASSOC modules in the first two articles; in the third one we relied on GESEE v1 (Tregouet & Tiret, Reference Tregouet and Tiret2000) as well as in SOLAR v4.0.1 (Almasy & Blangero, Reference Almasy and Blangero1998), whereas in the fourth study (Santos et al., Reference Santos, Katzmarzyk, Diego, Gomes, Santos, Blangero and Maia2014b), heritabilities and gene-by-environment interactions were exclusively computed in SOLAR v4.3.1 (Almasy & Blangero, Reference Almasy and Blangero1998). All of these parameter estimates were adjusted for sex, age, age2, age*sex, age2*sex, socioeconomic status and Body Mass Index (BMI) as needed.
Familial correlations for body composition are presented in Figure 1. For percentage of body fat, the lowest correlation was observed in spouses (−0.14) and the highest in siblings (0.28–0.34), but with some variation across studies. In Fermino et al. (Reference Fermino, Seabra, Garganta, Valdivia and Maia2008), siblings were divided by sib-type (brother–brother, sister–sister and brother–sister) whereas in de Chaves et al. (Reference de Chaves, Baxter-Jones, Santos, Gomes, dos Santos, de Souza and Maia2014) they were not, and we identified a similar trend in percentage of trunk fat such that the lowest correlations were in spouses (−0.15) and the highest for mother–daughter and brother–brother pairs (0.74). Heritability estimates in body composition phenotypes also varied across studies (Figure 2), ranging from .25 to .39 and .21 to .50 in percentage of body fat and percentage of trunk fat, respectively. Additionally, in Santos et al. (Reference Santos, Katzmarzyk, Diego, Gomes, Santos, Blangero and Maia2014b), total daily energy expenditure (TDEE; kcal/kg) and daily energy expenditure (DEE; kcal) in body composition genetic regulation were tested using a gene-by-environment interactions (G × TDEE and G × DEE). Results revealed significant interactions with all body composition phenotypes, thus indicating an interindividual variability in body composition expression, explained to some extent by an interaction between genotype and energy expenditure.
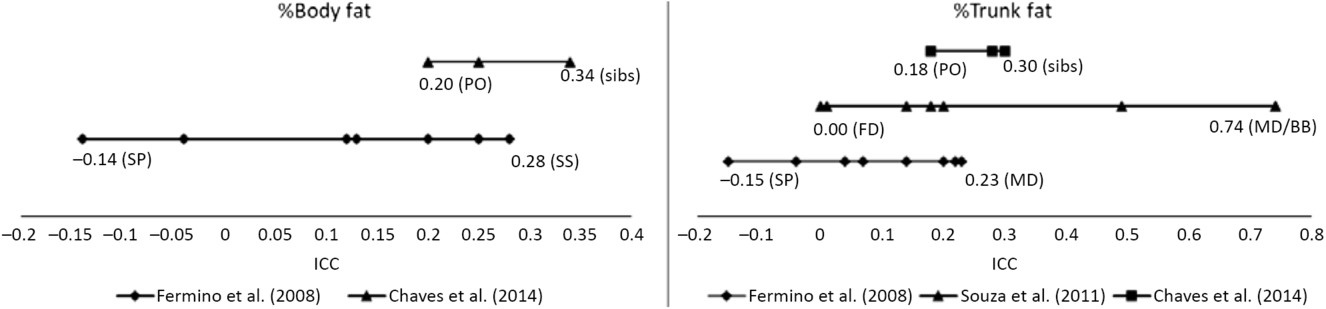
Fig. 1. Ranges of familial intraclass correlations coefficients (ICC) for body composition phenotypes.
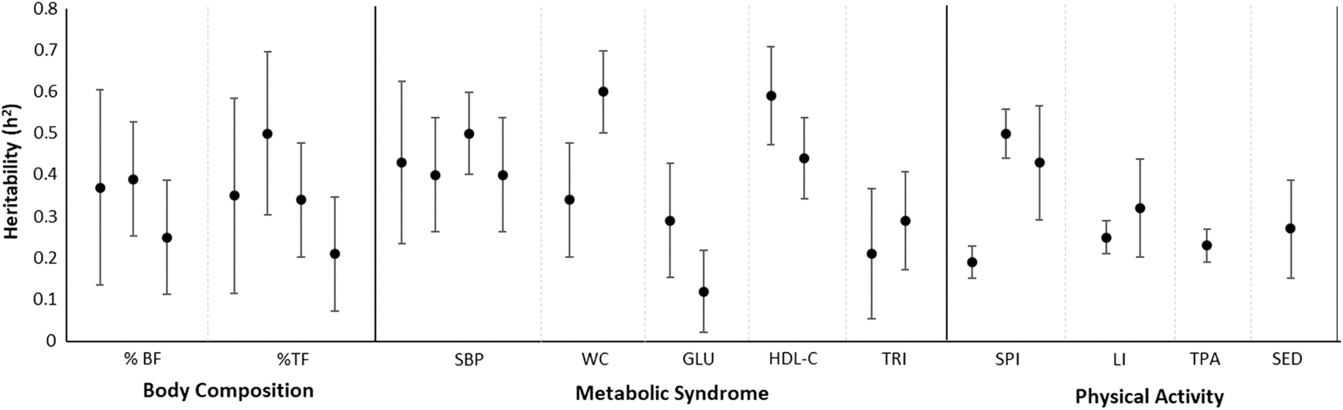
Fig. 2. Heritability estimates and corresponding 95% confidence intervals for phenotypes in all study domains.
In summary, low-to-moderate resemblance among family members in body composition traits was observed, meaning that it is important to design effective and creative programs within the family orbit to especially prevent increases in body fat, given its known links to several diseases like cardiovascular disease (Despres, Reference Despres2012), insulin resistance (Patel & Abate, Reference Patel and Abate2013), type 2 diabetes (Kleinert et al., Reference Kleinert, Clemmensen, Hofmann, Moore, Renner, Woods and Tschöp2018), hypertension (Chen et al., Reference Chen, Liang, Zheng, Wang and Lu2018) and metabolic syndrome (Kwon et al., Reference Kwon, Kim and Kim2017). Moreover, the significant interaction between genotype and energy expenditure in the expression of body traits was observed. Thus, a major point to note here is that physical activity promotes interindividual differences in body composition traits by genetic mediation. We foresee that future genetic research using top-down approaches with nuclear families, or even with sib-ships, should consider putative links of moderate-to-vigorous physical activity, food consumption behaviors and objective food eating habits, socioeconomic status, multiplicity of built environments and cultural diversity, together with various body composition phenotypes within a longitudinal perspective.
Metabolic Syndrome
Metabolic syndrome is defined as a constellation of interrelated pathophysiological risk factors, namely central adiposity, insulin resistance, dyslipidemia and hypertension, which increases the risk for cardiovascular diseases and type 2 diabetes (Alberti et al., Reference Alberti, Zimmet and Shaw2006). Over the years, research has been marked by several attempts to develop criteria to define accurate cut-points in each of the metabolic syndrome markers so that precise prevalence estimates could be provided (Alberti et al., Reference Alberti, Zimmet and Shaw2006). As metabolic syndrome is a forerunner to several noncommunicable diseases (Eckel et al., Reference Eckel, Grundy and Zimmet2005) there has also been an increased interest in the study of these clustered risk factors in children and adolescents, given the observed increase in its prevalence within the pediatric population.
As most of our data collection was done in field settings, we relied on standardized protocols and validated instruments used to measure metabolic syndrome indicators. For example, systolic blood pressure was assessed using three consecutive measures (with a 2 or 3 min’s interval between them) with an automatic digital Omron sphygmomanometer (Omron M6, hem 7001-E, Omron Healthcare); a finger-stick blood sample was collected to obtain high-density lipoprotein cholesterol (HDL-cholesterol), triglycerides and glucose using the Cholestech LDX point of care device (Cholestech Corporation, Hayward, CA, USA); finally, waist circumference was measured using a nonelastic anthropometric tape (Sanny, American Medical of Brazil, Brazil).
Three studies have data on all metabolic syndrome markers — one study relied on a sample of siblings, whereas the other two included nuclear families. Furthermore, two other studies only used blood pressure indicators. Sample sizes varied from 107 to 515 nuclear families and the sibling study sampled 333 sibling pairs (Pereira et al., Reference Pereira, Katzmarzyk, Gomes, Buranarugsa, Moura-Dos-Santos, Hedeker and Maia2019). All studies adjusted their statistical analysis for several covariates — Santos et al. (Reference Santos, Katzmarzyk, Diego, Souza, Chaves, Blangero and Maia2013a) and (Reference Santos, Katzmarzyk, Tregouet, Gomes, Santos and Maia2013b) used age2, sex, age*sex, and age2*sex, whereas Fermino et al. (Reference Fermino, Seabra, Garganta and Maia2009) used age, sex, age2, age3, age*sex, age2*sex and BMI; further, de Chaves et al. (Reference de Chaves, Baxter-Jones, Santos, Gomes, dos Santos, de Souza and Maia2014) used sex, age, age2, age*sex, age2*sex and Socioeconomic status (SES), while Pereira et al. (Reference Pereira, Katzmarzyk, Gomes, Buranarugsa, Moura-Dos-Santos, Hedeker and Maia2019) used age2, BMI, maturity offset, fruit and vegetable consumption, sugary drink consumption, TPAy and muscular fitness and cardiorespiratory fitness scores.
Siblings showed higher similarity than other family members in all metabolic syndrome markers, except for glucose in the Santos et al. (Reference Santos, Katzmarzyk, Tregouet, Gomes, Santos and Maia2013b) study; where spousal resemblance was higher than sibling resemblance. The lowest familial similarity was found for glucose and triglycerides (heritability [h 2] estimates lower than .30; Figure 3). The remaining markers revealed moderate h 2, with values ranging from .40 for systolic blood pressure to .60 for waist circumference. The heritability was lower in this study compared with that reported by Santos et al. (Reference Santos, Katzmarzyk, Diego, Souza, Chaves, Blangero and Maia2013a; h 2 = .34). These discrepancies may be linked to differences in sample sizes and to differences in family compositions, that is, different kin structure among studies. In any case, these studies provide a significant body of results regarding the influence of genetic and environmental factors on metabolic syndrome Therefore, when considering the intertwined effects of factors regulating metabolic syndrome expression, we believe that future family studies are needed to investigate putative associations between metabolic syndrome and objective physical activity measures, as well as physical fitness levels, nutritional behaviors and objective food consumption using longitudinal designs integrating top-down and bottom-up methodologies to better understand the complex works of nature and nurture on metabolic syndrome.

Fig. 3. Ranges of familial intraclass correlations coefficients (ICC) for physical activity phenotypes.
Physical Activity and Sedentary Behavior
Physical activity can be defined as any bodily movement produced by skeletal muscles that results in energy expenditure (Caspersen et al., Reference Caspersen, Powell and Christenson1985). Previous research reported that adequate physical activity levels reduce the incidence of several noncommunicable diseases and premature mortality (Lee et al., Reference Lee, Shiroma, Lobelo, Puska, Blair and Katzmarzyk2012). On the other hand, high levels of sedentary behaviors (e.g. TV viewing, video game playing and computer use) have been shown to negatively impact health-related outcomes, regardless of the influence of other important factors such as diet and physical activity (de Rezende et al., Reference de Rezende, Rodrigues Lopes, Rey-López, Matsudo and Luiz Odo2014).
Our Portuguese research studies have had a special focus on studying physical activity and sedentary behavior using family data (Table 4). Sample sizes ranged from 206 to 3378 nuclear families and, as expected in large studies, we mostly relied on a simple method to assess physical activity — the Baecke questionnaire (Baecke et al., Reference Baecke, Burema and Frijters1982) with its three domains: work/school physical activity, leisure-time physical activity and sport participation; further, a measure of TPA was generated by summing the individual scores in these domains. Sedentary behavior was obtained from three different questionnaires: The International Physical Activity questionnaire (Television viewing; Bassett, Reference Bassett2003); the 3-day physical activity diary (minutes spent in sedentary behaviors and energy expenditure in sedentary; Bouchard et al., Reference Bouchard, Tremblay, Leblanc, Lortie, Savard and Theriault1983); the Baecke questionnaire (Physical Activity tertiles), namely the total time the subject used a personal computer per day (personal computer usage).
Further, different studies used different analytic software based on specific aims. For example, FCOR and ASSOC modules (Pereira et al., Reference Pereira, Katzmarzyk, Gomes, Elston and Maia2018a; Seabra et al., Reference Seabra, Mendonca, Goring, Thomis and Maia2008, Reference Seabra, Mendonca, Goring, Thomis and Maia2014), GESEE and SOLAR (de Chaves et al., Reference de Chaves, Baxter-Jones, Santos, Gomes, dos Santos, de Souza and Maia2014; Maia et al., Reference Maia, Gomes, Tregouet and Katzmarzyk2014; Santos et al., Reference Santos, Katzmarzyk, Diego, Blangero, Souza, Freitas and Maia2014a), and multilevel models using SPSS and SuperMix (Gomes et al., Reference Gomes, Dos Santos, Garganta, Kenny, Katzmarzyk and Maia2014; Pereira et al., Reference Pereira, Katzmarzyk, Gomes, Souza, Chaves, Santos and Maia2018b) were used. Similarly, adjusting for covariates was also different. For example, de Chaves et al. (Reference de Chaves, Baxter-Jones, Santos, Gomes, dos Santos, de Souza and Maia2014), Maia et al. (Reference Maia, Gomes, Tregouet and Katzmarzyk2014), Pereira et al. (Reference Pereira, Katzmarzyk, Gomes, Elston and Maia2018a), Seabra et al. (Reference Seabra, Mendonca, Goring, Thomis and Maia2008, Reference Seabra, Mendonca, Goring, Thomis and Maia2014) and mostly adjusted for biological factors, whereas Pereira et al. (Reference Pereira, Katzmarzyk, Gomes, Souza, Chaves, Santos and Maia2018b) considered the joint effects of biological, behavioral and environmental characteristics.
Figure 2 shows familial/sibling correlations for TPA, leisure time and sports participation, and the results reveal a similar trend: father–daughter pairs have the lowest resemblance, and sister–sister pairs had the highest resemblance for TPA. For the leisure-time physical activity index, mother–son and father–son pairs had the lowest resemblance while brother–brother and brother–sister pairs had the highest resemblance. For the sports participation index (SPI), mother–son pairs had the lowest resemblance, whereas siblings had the highest. Heritability estimates for the three domains were generally low, ranging from .23 for TPA to .32 for the leisure-time physical activity index. Additionally, h 2 estimates for sedentary behavior phenotypes were also low — from .04 for daily computer use to .27 in sedentary time. Additionally, Pereira et al. (Reference Pereira, Katzmarzyk, Gomes, Elston and Maia2018a) examined the stability of genetic factors for TPA and SPI from early (10–14 years) to late adolescence (15–19 years), and no significant differences were found across estimates: h 2 = .297 and .322, h 2 = .413 and .428, for TPA and SPI, respectively.
In summary, our results showed a moderate genetic influence on physical activity and sedentary behavior. Additionally, heritability tends to remain stable from early to late adolescence. Further, the propensity to be physically active and/or sedentary is mostly determined by the interaction between genetic endowments and shared environmental characteristics. These findings provide a fertile ground for novel family research opportunities, longitudinally based, and using more detailed information on the specificities of shared and unique environments linked to physical activity and sedentariness. These have to link quantitative as well as qualitative information and probe into the stability, or change, of these different environments.
Health-Related Physical Fitness
Broadly speaking, physical fitness is an individual attribute that allows a person to carry out his/her daily activities without undue fatigue and with adequate energy reserves to enjoy leisure pursuits (Malina & Katzmarzyk, Reference Malina and Katzmarzyk2006). It has been suggested that physical fitness is an important health marker (Ortega et al., Reference Ortega, Cadenas-Sanchez, Lee, Ruiz, Blair and Sui2018), and there is growing evidence showing that adequate physical fitness levels are associated with the prevention of overweight during childhood and adolescence (Rodrigues et al., Reference Rodrigues, Stodden and Lopes2016), with cognitive development (Haapala Reference Haapala2013), as well as academic achievement (Eveland-Sayers et al., Reference Eveland-Sayers, Farley, Fuller, Morgan and Caputo2009).
Our physical fitness studies relied on samples of siblings (Table 5). Vasques et al. (Reference Vasques, Lopes, Seabra, Fermino and Maia2007) used the following tests: curl-up, push-up, trunk-lift and 1-mile run/walk in a sample of 366 sibs (732 subjects) aged 10–18 years, whereas Pereira et al. (Reference Pereira, Todd Katzmarzyk, Gomes, Souza, Chaves, Dos Santos and Maia2017b) used 540 sibs (1101 subjects) aged 9–20 years, and relied on the following tests: waist circumference, percentage of body fat, handgrip strength, standing long jump, 50-yard dash, shuttle run and 1-mile run. In addition, statistical procedures used for data analysis also varied across the different studies. Vasques et al. (Reference Vasques, Lopes, Seabra, Fermino and Maia2007) used Pearson and canonical correlations controlling for age, while Pereira et al. (Reference Pereira, Todd Katzmarzyk, Gomes, Souza, Chaves, Dos Santos and Maia2017b) estimated intraclass correlation coefficients from multilevel models after adjustment for a number of covariates, including age, age2, maturity offset, TPA, TV viewing, socioeconomic status and a series of interactions of age with sib-types.
In both studies, sibling similarity was specific to each physical fitness test and depended on the statistical adjustments made for biological, behavioral and environmental characteristics. However, in general, a similar pattern emerged: same-sex siblings showed the greatest resemblance for all physical fitness tests. For example, Vasques et al. (Reference Vasques, Lopes, Seabra, Fermino and Maia2007) reported that sibling similarity ranged from .22 (curl-up) to .49 (1-mile run/walk) in same-sex siblings, and from .02 (trunk-lift) to .14 (curl-up) in opposite-sex siblings. Further, Pereira et al. (Reference Pereira, Todd Katzmarzyk, Gomes, Souza, Chaves, Dos Santos and Maia2017b) reported that resemblance varied from .11 (1-mile run/walk) to .54 (handgrip strength) in same-sex siblings, while in opposite-sex siblings no resemblance was found in unadjusted intraclass correlations for all fitness tests. Yet, when the model was fully adjusted, the highest resemblance value (.14) was observed for the 1-mile run/walk.
Overall, these results highlight the importance of explaining interindividual variation in physical fitness levels considering the combined effects of genetic factors as well as shared and unique environmental factors. Future research should use longitudinal nuclear family data (or using only sib-ships) to examine this complex web of putative links (body composition, physical activity and sedentariness) that may influence physical fitness changes over time.
Summary
The studies we have conducted under the umbrella of the Portuguese Healthy Family Study have resulted in several important results:
1. Sibling similarity in somatotype is moderate and consistent among the sibling studies, suggesting a degree of genetic regulation of body shape.
2. Resemblance in body composition phenotypes is moderately influenced by genetic factors. Energy expenditure interacts with genotype promoting a substantial degree of heterogeneity in body composition.
3. Glucose and triglycerides demonstrate the least familial similarity, whereas waist circumference, blood pressure and HDL-cholesterol showed moderate resemblance. These results shed light into the complex intertwined effects of genes and the environment in the expression of metabolic syndrome in family members.
4. Familial resemblance (shared genes and environments) in physical activity domains (TPA, sports index and leisure time) as well as in sedentary behaviors was low. Further, genetic factors regulating leisure-time physical activity as well as sports index were stable across adolescence.
5. Sibling resemblance in physical fitness is specific to each fitness test. Further, same-sex siblings are substantially more alike than opposite-sex siblings.
Acknowledgements
The authors express their gratitude to all the participants in the Portuguese Healthy Family Study.
Financial support
This work was supported by the FCT — Foundation for Science and Technology. The authors thank them for granting this research (PTDC/DES/67569/2006 and FCOMP-01-0124FEDEB-09608).
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.










