Introduction
The German river Weser along with its tributary Werra has a long history of salt pollution. Since the early 20th century, discharge of waste waters by the local potash mining industry into the Werra severely increased the river's salinity (Braukmann and Böhme, Reference Braukmann and Böhme2011; Schulz and Cañedo-Argüelles, Reference Schulz and Cañedo-Argüelles2019). Subsequently, the decrease in water quality led to an impoverished diversity of macrozoobenthic organisms (Hübner, Reference Hübner2007). To replace the disappeared indigenous Gammarus species, the euryhaline Gammarus tigrinus Sexton, 1939 was intentionally released into the Werra by Schmitz in 1957. This North American amphipod species quickly established a dense population, spreading beyond the Weser–Werra–Estuary into other river systems and the Baltic Sea (Bulnheim, Reference Bulnheim1976; Rewicz et al., Reference Rewicz, Grabowski, Tończyk, Konopacka and Bącela-Spychalska2019; Spikkeland et al., Reference Spikkeland, Olsen, Kasbo, Olsen and Nilssen2020).
The first infections of G. tigrinus with the acanthocephalan Paratenuisentis ambiguus (Van Cleave, 1921) in the Weser were discovered a few decades after the initial release of the alien amphipod (Taraschewski et al., Reference Taraschewski, Moravec, Lamah and Anders1987). In its recipient range as well as its native area, P. ambiguus has been recorded from G. tigrinus as well as the indigenous eel species Anguilla anguilla (Linnaeus, 1758) and Anguilla rostrata (Lesuer, 1817) as final host (Gilbert and Bullock, Reference Gilbert and Bullock1981; Taraschewski et al., Reference Taraschewski, Moravec, Lamah and Anders1987).
In comparison, species of Pomphorhynchus have been recorded from a variety of intermediate and definitive hosts. However, the data available are masked by the puzzling taxonomy of this acanthocephalan genus in Europe. Nowadays, the Pomphorhynchus species complex in Central Europe is considered to comprise at least 3 species, Pomphorhynchus tereticollis (Rudolphi, 1809), Pomphorhynchus laevis (Zoega in Müller, 1776) and Pomphorhynchus bosniacus Kiskároly & Čanković, 1969. These congeners can be well distinguished on a molecular level but express a somewhat similar polymorphic morphology that may have led to misidentifications in the past (Špakulová et al., Reference Špakulová, Perrot-Minnot and Neuhaus2011; Perrot-Minnot et al., Reference Perrot-Minnot, Špakulová, Wattier, Kotlík, Düşen, Aydoğdu and Tougard2018; Reier et al., Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019).
After a long period of taxonomic dis- and reassembling, P. tereticollis was conclusively reestablished as distinguishable from P. laevis (Špakulová et al., Reference Špakulová, Perrot-Minnot and Neuhaus2011). Several studies focused on the replacement of these Pomphorhynchus species by each other, following the recent range expansion of their respective intermediate and paratenic hosts (Emde et al., Reference Emde, Rueckert, Palm and Klimpel2012; David et al., Reference David, Staentzel, Schlumberger, Perrot-Minnot, Beisel and Hardion2018; Hohenadler et al., Reference Hohenadler, Nachev, Thielen, Taraschewski, Grabner and Sures2018). However, no differentiation between P. bosniacus and P. laevis was made in these studies, as the distinctiveness of both species had not yet been revealed (Reier et al., Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019). Furthermore, information on the host–parasite relationship of previous invaders, such as G. tigrinus, became scarce, as most authors focused on the Ponto-Caspian invasion wave of amphipods, especially Dikerogammarus villosus (Sowinsky, 1894) (Daunys and Zettler, Reference Daunys and Zettler2006; Kornis et al., Reference Kornis, Mercado-Silva and vander Zanden2012; Reisalu et al., Reference Reisalu, Kotta, Herkül and Kotta2016; MacNeil, Reference MacNeil2019). Until now, G. tigrinus had not been reported to serve as an intermediate host of a Pomphorhynchus species in Europe. In its native area of North America, G. tigrinus is parasitized by Pomphorhynchus rocci Cordonnier & Ward, Reference Cordonnier and Ward1967 (Johnson and Harkema, Reference Johnson and Harkema1971). However, thus far P. rocci failed to colonize European populations of G. tigrinus.
In addition to the species debate within the Pomphorhynchus genus, the taxonomic resolution of other acanthocephalans, such as Polymorphus minutus (Goeze, 1782), is equally intricate. This parasite of waterbirds may share some intermediate hosts, such as Gammarus pulex (Linnaeus, 1758), with Pomphorhynchus spp. (Kaldonski et al., Reference Kaldonski, Perrot-Minnot, Motreuil and Cézilly2008). However, recent advances in phylogenetic research led to the emergence of cryptic species of P. cf. minutus that differ greatly in their intermediate host specificity (Zittel et al., Reference Zittel, Grabner, Wlecklick, Sures, Leese, Taraschewski and Weigand2018; Grabner et al., Reference Grabner, Doliwa, Bulantová, Horák and Sures2020).
Since the intentional release of G. tigrinus in the Weser river system, the arrival of other alien species such as D. villosus increased the local xenodiversity, as these non-indigenous species now account for the largest proportion of the macrozoobenthic community composition (Grabow et al., Reference Grabow, Eggers and Martens1998). After the initial studies on host–parasite relationships by Taraschewski et al. (Reference Taraschewski, Moravec, Lamah and Anders1987) little to no efforts have been made to evaluate the recent parasitical developments in this system.
To address the informational gap, we investigated gammarids and their acanthocephalan parasites in the river Werra. After the discovery of P. tereticollis in G. tigrinus, the main objective of this study was to compare the intermediate host patterns of acanthocephalans in the Weser river system. Regarding the challenging taxonomy of Pomphorhynchus a combination of biological, morphological and phylogenetic means of differentiation was chosen. For non-indigenous acanthocephalans, whether their range extension was driven by host–parasite co-invasion or by host capture is discussed.
Materials and methods
Sampling
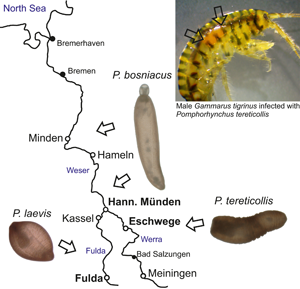
Six sites were chosen for gammarid acquisition between 2018 and 2021 (Fig. 1). The sites consist of the Werra in Eschwege (51°11′28.2″N, 10°03′24.9″E) and Meiningen (50°34′04.5″N, 10°24′33.2″E), the Fulda in the cities Fulda (50°33′16.3″N, 9°39′52.0″E) and Kassel (51°17′20.2″N, 9°29′25.2″E) and the Weser near Hannoversch Münden (51°28′07.7″N, 9°38′42.1″E) and Hameln (52°06′32.0″N, 9°20′59.3″E). Amphipods were captured via kick-sampling using a hand sieve (2 mm mesh width), transported to the laboratory alive and deep-frozen until dissection. The sex, body length from head to urosome excluding the uropod, length of the second antennae as well as the infection status were recorded for each gammarid. In most cases, infected specimens could be easily recognized by the orange-red colour of the cystacanth (Fig. S1a). Prevalence [P (%)] as well as mean intensity of infection (MI) were calculated for each sample (Table 1). To enhance passive proboscis evagination, cystacanths from defrost amphipods were maintained overnight in distilled water at 8°C. Photographs of whole, unfixed specimens were taken using a Kayence VHX-7000 digital microscope. The samples were then preserved in 95% ethanol for molecular analysis.

Fig. 1. Location of the study sites along the Weser river system in northern Germany (full circles).
Table 1. Amphipod populations infected with Pomphorhynchus spp., according to sampling month and number of (infected) hosts per sample.

P (%): prevalence; MI: mean intensity of infection.
1 Eschwege.
2 Hann. Münden.
3 Fulda.
In addition, adult specimens of P. bosniacus were obtained by dissecting the intestines of frozen eels gathered from local fishermen in Nienburg and Landesbergen. We were assured that all fish originated from the river Weser. Specimens obtained from the dissections were stored in 95% ethanol until molecular analysis.
Morphological identification
Paratenuisentis ambiguus (Fig. S1b) was identified based on its original description and subsequent records from the Weser river system (Bullock and Samuel, Reference Bullock and Samuel1975; Taraschewski et al., Reference Taraschewski, Moravec, Lamah and Anders1987). Polymorphus cf. minutus was recognizable by its bright colour and surrounding envelope (Fig. S1c) (Dezfuli and Giari, Reference Dezfuli and Giari1999). A single specimen was used for DNA barcoding according to Zittel et al. (Reference Zittel, Grabner, Wlecklick, Sures, Leese, Taraschewski and Weigand2018). The morphological identification of Pomphorhynchus spp. (Fig. S1d–f) was performed using the keys available (Amin et al., Reference Amin, Abdullah and Mhaisen2003), as well as the criteria described by Špakulová et al. (Reference Špakulová, Perrot-Minnot and Neuhaus2011) and the observations from Reier et al. (Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019).
DNA barcoding
To ensure the correct taxonomic placement of Pomphorhynchus specimen, a DNA barcoding approach was chosen. DNA was extracted using Chelex® and amplification of cytochrome c oxidase subunit I (COI) was performed according to Tierney et al. (Reference Tierney, Caffrey, Vogel, Matthews, Costantini and Holland2020). Polymerase chain reaction products were sent to Microsynth Seqlab for Sanger sequencing using the forward primer. Ninety-five sequences of 507 bp length were obtained: 73 P. tereticollis from G. tigrinus; 12 P. bosniacus from A. anguilla, 2 from D. villosus and 8 P. laevis from G. pulex. Initial alignment was performed using ClustalW in MEGA 11 (Kumar et al., Reference Kumar, Stecher, Li, Knyaz and Tamura2018), before constructing median-joining (MJ) haplotype networks (Bandelt et al., Reference Bandelt, Forster and Röhl1999) in PopART (https://popart.maths.otago.ac.nz/) (Leigh and Bryant, Reference Leigh and Bryant2015) (Fig. 2).

Fig. 2. MJ haplotype network constructed with PopART containing 95 sequences obtained in this study. * indicate possible pseudogenes.
For further phylogenetic analysis, the following NCBI accessions were added to the dataset EF051062–EF051071 (Moret et al., Reference Moret, Bollache, Wattier and Rigaud2007), JN695505–JN695508 (Špakulová et al., Reference Špakulová, Perrot-Minnot and Neuhaus2011), JQ824373 (Pan and Nie, 2012, unpublished), KJ819957–KJ820006 (Vardić Smrzlić et al., Reference Vardić Smrzlić, Valić, Kapetanović, Filipović Marijić, Gjurčević and Teskeredžić2015), KY075794 (Andreou et al., Reference Andreou, Antognazza, Williams, Bradley, Reading, Hardouin, Stewart, Sheath, Galligar, Johnson and Britton2020), KY490045–KY490047 (Li et al., Reference Li, Chen, Amin and Yang2017), KY911293–KY911323 (García-Varela et al., Reference García-Varela, Mendoza-Garfias, Choudhury and Pérez-Ponce de León2017), LN994840–LN994853 (Perrot-Minnot et al., Reference Perrot-Minnot, Špakulová, Wattier, Kotlík, Düşen, Aydoğdu and Tougard2018), MF563495–MF563527 (David et al., Reference David, Staentzel, Schlumberger, Perrot-Minnot, Beisel and Hardion2018), MK133340–MK133344 (Nedić et al., Reference Nedić, Vardić Smrzlić, Paraš and Nikolić2019) and MK612497–MK612545 (Reier et al., Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019), MT216151–MT216172 (Ros et al., Reference Ros, Basen, Teschner and Brinker2020) (for more details see the Supplementary material). The total dataset of 316 sequences was edited in MEGA 11 and trimmed to a length of 507 bp. An MJ haplotype network was constructed for each Pomphorhynchus lineage (Fig. S2a–c). We chose a subset of 33 sequences to construct a neighbour-joining (NJ) tree in MEGA 11 using uncorrected P-distances (Fig. 3). Branch support was assessed with 1000 bootstrap replicates and partial deletion was chosen as missing data treatment to accommodate for sequences shorter than 507 bp.

Fig. 3. NJ phylogenetic tree computed using MEGA 11. Numbers indicate branch support assessed by 1000 bootstrap iterations (values below 50 are not shown). Titles consist of NCBI accession number, sampling location and host (if available). Coloured markers indicate sequences obtained in this study. * indicate potential pseudogenes or mtDNA-like sequences.
Results
Amphipod distribution
For each of the sampled river sections, a distinct community composition of Amphipoda was observed (Table 2). In the Werra section impacted by high salinity, G. tigrinus was the main component of the macrozoobenthic community (n = 4329). Upstream of the salt discharge sites in the Werra, only a small abundance of gammarids was encountered. The few amphipods obtained (n = 22) belonged to Gammarus roeselii (Gervais, 1835).
Table 2. Distribution of amphipods and acanthocephalans in the investigated area

N: total number of amphipods; N inf: number of infected hosts; P (%): prevalence; MI: mean intensity of infection.
1 Werra.
2 Weser.
3 Fulda.
The Weser at Hannoversch Münden was populated by a quantity of D. villosus (n = 866), in addition to single records of Chelicorophium curvispinum (Sars, 1895), G. pulex and G. roeselii. Similarly, the sampling site in Hameln was dominated by D. villosus (n = 167). Here, 2 specimens of G. tigrinus were recorded outside the river Werra. The lower Fulda at Kassel was not distinct from the Weser samples, as only D. villosus was found here (n = 1300). More upstream, the river Fulda at the city Fulda was inhabited by G. pulex (n = 1361) and G. roeselii (n = 45).
Parasite distribution
Paratenuisentis ambiguus was found in low prevalence (0.2 ± 0.5%) in G. tigrinus from the Werra throughout the sampling period and only a small quantity of adults (n = 3) could be obtained from the required definitive host A. anguilla in the Weser in 2018. General prevalence of P. tereticollis in G. tigrinus in the river Werra was different when comparing total [P (%): 3.2] and mean prevalence (6.1 ± 6.6%), but specimens were found throughout the investigation period (Table 1). During sampling in 2019, 2 cystacanths of waterbird-parasitizing P. cf. minutus were also discovered in G. tigrinus. However, prevalence in the single sample was low (0.2%) and no further infections could be recorded.
Four specimens of D. villosus harboured P. bosniacus, although prevalence in the intermediate host was generally low [P (%): 0.5]. However, the intestinal parasite community of the eel investigated (n = 48) was dominated by subadult P. bosniacus [P (%): 72.9; MI: 5.7], of which only 19.9% truly penetrated the intestinal wall while the rest were merely attached to it. No gravid females were discovered.
Pomphorhynchus laevis was collected from G. pulex in small abundance during samplings of the Fulda in 2020, where prevalence ranged between 0.7 and 5.8, with a total prevalence of 1.4.
Morphological identification
Cystacanths of Pomphorhynchus spp. showed a different shape (Fig. S1d–f) and proboscis hook formula depending on river section of origin and intermediate host, respectively. Pomphorhynchus tereticollis had an elongated, cylindrical trunk with a wrinkled surface in most, though not all larvae (Fig. S1d), and a proboscis armed with 14–20 longitudinal rows of 9–12 hooks each. Length of the relaxed cystacanths ranged between 2.9 and 4.9 mm. The few specimens obtained from the Weser section near Hannoversch Münden (Fig. S1e) were of similar shape and length (3.5 mm) but had a smoother trunk and fewer hooks (7–9) in 14 longitudinal rows, therefore they were identified as P. bosniacus. In comparison, P. laevis larvae from the river Fulda (Fig. S1f) were of a more compressed, ovoid or spindle-like shape, smaller overall trunk size (1.0–2.9 mm) and armed with 16–20 rows of 10–13 hooks each.
Phylogenetic analysis
A total of 83 cystacanths (2 P. bosniacus, 8 P. laevis, 73 P. tereticollis) and 12 subadult specimens (P. bosniacus) were used for phylogenetic analysis. All sequences obtained in this study clustered according to sampling location and host, thus confirming our morphological species identification. Four haplogroups could be differentiated in our dataset (Fig. 2), each of the 3 Pomphorhynchus species being represented by 1, in addition to a 4th haplogroup in between the main clusters of P. bosniacus and P. laevis. Mean genetic P-distance between this haplogroup and P. bosniacus is 0.084 ± 0.013, and 0.073 ± 0.01 between it and P. laevis, respectively. Distance between P. bosniacus and P. laevis in our samples is 0.112 ± 0.013. In the NJ tree (Fig. 3), the 3 sequences in this additional cluster appear closer to P. laevis, however, their placement is not supported.
Discussion
Amphipod distribution
Sampling the main streams of the Weser river system provided a characteristic gammarid community for each Werra, Fulda and Weser. Gammarus tigrinus was the main macrozoobenthic component in the Werra, and seems to be the only amphipod, besides Apocorophium lacustre (Vanhöffen, 1911), to inhabit the polluted river sections (Szöcs et al., Reference Szöcs, Coring, Bäthe and Schäfer2014). Our results suggest G. tigrinus almost completely disappeared from the Weser, although it reached high abundances in the northern central Europe as well as the Baltic Sea following its introduction to Europe (Spikkeland et al., Reference Spikkeland, Olsen, Kasbo, Olsen and Nilssen2020). It was replaced by D. villosus that had been reported from the investigation area more than 20 years ago (Grabow et al., Reference Grabow, Eggers and Martens1998). This Ponto-Caspian super-spreader, along other newly introduced amphipods, extended its range into central Europe following the inauguration of the Rhine–Main–Danube Canal (Grabow et al., Reference Grabow, Eggers and Martens1998; Leuven et al., Reference Leuven, van der Velde, Baijens, Snijders, van der Zwart, Lenders and Bij de Vaate2009; Rewicz et al., Reference Rewicz, Grabowski, MacNeil and Bącela-Spychalska2014). Considering its invasive potential, it has likely outcompeted G. tigrinus in German rivers, including the Weser. However, data from the Netherlands show that G. tigrinus can compete with the highly competitive D. villosus by retreating to deeper regions (Platvoet et al., Reference Platvoet, Dick, MacNeil, van Riel and van der Velde2009). Only shallow shores are accessible for kick-sampling, therefore, our results regarding the distribution of G. tigrinus in the Weser may be prone to sampling bias.
Although D. villosus successfully extended its range across the Weser into the upper Fulda, it has not colonized the Werra. Therefore, the fusion of Fulda and Werra forming the Weser at Hannoversch Münden likely acts as a salinity barrier for D. villosus, preventing its spread (Boets et al., Reference Boets, Lock, Messiaen and Goethals2010; Gallardo et al., Reference Gallardo, Errea and Aldridge2012). With the emergence of the new xenodiversity in the Weser river system, native gammarids have been largely replaced. In the upper Fulda, G. pulex can still be found, although it does not seem to inhabit the river Weser anymore. The species has also been reported from unpolluted parts of the Werra (Braukmann and Böhme, Reference Braukmann and Böhme2011).
Paratenuisentis ambiguus
Host–parasite interactions in the river Weser and its tributary Werra appear to be highly influenced by ecological globalization, as revealed by the 5 acanthocephalan species present in this study. About 35 years ago, the successful jump invasion of the American eel-specific acanthocephalan P. ambiguus was discovered, when it adopted the European eel as a novel final host and retained the American amphipod G. tigrinus as its intermediate host (Taraschewski et al., Reference Taraschewski, Moravec, Lamah and Anders1987). Gammarus tigrinus was intentionally released into the Werra in 1957, after the indigenous gammarid fauna disappeared due to salinization by potash mining effluents (Schmitz, Reference Schmitz1960; Bulnheim, Reference Bulnheim1976). The introduced specimens were raised in a laboratory; therefore, it is unlikely that P. ambiguus invaded the habitat with its intermediate host. Supporting this assumption, there usually exists a lag of one to several decades between colonization by a novel host and the arrival of its parasite (Taraschewski, Reference Taraschewski2006).
As shown in this study, the natural acanthocephalan parasite of G. tigrinus, P. ambiguus, is still reminiscent in the Weser river system, although drastically declined in comparison to the previously reported high abundance (Taraschewski et al., Reference Taraschewski, Moravec, Lamah and Anders1987). Paratenuisentis ambiguus has been reported from G. tigrinus in the river Rhine, coastal waters of Poland and the northern Vistula Lagoon in Russia (Sures et al., Reference Sures, Knopf, Würtz and Hirt1999; Rodjuk and Shelenkova, Reference Rodjuk and Shelenkova2006; Dzido et al., Reference Dzido, Rolbiecki, Izdebska and Bednarek2020), but to our best knowledge there are no other records of this acanthocephalan in Europe. A contributing factor to the narrow ecological amplitude of this brackish water-transmitted acanthocephalan might be its dependence on eels and on G. tigrinus. The jump invasion into Europe coincided with the host capture of A. anguilla, but thus far no intermediate host other than G. tigrinus has been reported to be infected with P. ambiguus, neither in North America nor in the novel area Europe.
Pomphorhynchus
Other genera of Acanthocephala reveal a much more complicated taxonomy related to their invasion ecology. For several decades, P. tereticollis was considered synonymous with P. laevis until becoming reestablished based on morphological as well as molecular data (Špakulová et al., Reference Špakulová, Perrot-Minnot and Neuhaus2011). In contrast, the original description of P. bosniacus did not gain much response in literature and until its recent revival based on a combined morphological and genetic approach, this species was potentially misidentified as P. laevis in Central Europe (Kiskároly and Čanković, Reference Kiskároly and Čanković1967; Reier et al., Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019).
Two lineages of Pomphorhynchus, herein referred to as P. bosniacus and P. laevis were identified in the Weser river system. The results presented in this study demonstrate that those 2 lineages differ greatly in their utilization of intermediate hosts in the Weser system, with P. laevis being found in G. pulex in the Fulda and P. bosniacus in D. villosus in the Weser, respectively. The distinction in the life cycle of both these acanthocephalans further affirms their consideration as 2 different species.
Additionally, the anatomical features of the Fulda and Weser specimen indicate a simple morphological distinction based on the formula of the proboscis hook armature might be possible, as P. bosniacus possessed fewer hooks per row than its congeners. On the contrary, cystacanths of P. laevis were of smaller size and more ovoid to spindle-like shape when compared to P. bosniacus and P. tereticollis. Differences in the morphology of cystacanths within the Pomphorhynchus species complex have been described previously (Perrot-Minnot, Reference Perrot-Minnot2004), but were never utilized to a broader application as an additional criterion for species identification.
Inconsistencies in terms of genetic relationships and therefore taxonomy within the genus Pomphorhynchus based on the COI locus have been reported in various studies (Perrot-Minnot et al., Reference Perrot-Minnot, Špakulová, Wattier, Kotlík, Düşen, Aydoğdu and Tougard2018; Nedić et al., Reference Nedić, Vardić Smrzlić, Paraš and Nikolić2019; Mauer et al., Reference Mauer, Hellmann, Groth, Fröbius, Zischler, Hankeln and Herlyn2020). Phylogenetic comparison of specimen from the Weser river system supports the existence of 3 separate species. Pomphorhynchus tereticollis in our sample was closely related to specimens from southern Germany and the British Isles (Andreou et al., Reference Andreou, Antognazza, Williams, Bradley, Reading, Hardouin, Stewart, Sheath, Galligar, Johnson and Britton2020; Ros et al., Reference Ros, Basen, Teschner and Brinker2020). Haplotype analysis and the NJ tree revealed that P. laevis belonged to the western-European clade previously described by Perrot-Minnot et al. (Reference Perrot-Minnot, Špakulová, Wattier, Kotlík, Düşen, Aydoğdu and Tougard2018). Most COI sequences of P. bosniacus fitted well within the invasive lineage that has extended its range across the rivers Danube and Rhine in the last decade (David et al., Reference David, Staentzel, Schlumberger, Perrot-Minnot, Beisel and Hardion2018; Reier et al., Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019). However, 3 specimens clustered with a single sequence (MK133341) from the river Sava (Nedić et al., Reference Nedić, Vardić Smrzlić, Paraš and Nikolić2019). Based on host and distribution range, as well as morphological features, it was not possible to distinguish between these 2 P. bosniacus haplogroups. Given several base substitutions when compared to other P. bosniacus and P. laevis in our dataset, it is likely that these sequences represent mtDNA-like sequences or pseudogenes (Vardić Smrzlić et al., Reference Vardić Smrzlić, Valić, Kapetanović, Filipović Marijić, Gjurčević and Teskeredžić2015; Nedić et al., Reference Nedić, Vardić Smrzlić, Paraš and Nikolić2019).
Co-invasion
Although infection rate in general is low, D. villosus serves as an intermediate host of P. bosniacus in the colonized river Weser in Germany. This is further highlighted by the abundance of subadults found in the eels investigated from Landesbergen and Nienburg in 2018. The parasite seems to have followed the trail of its highly expansive Ponto-Caspian amphipod, colonizing river systems in central, northern and western Europe (Rewicz et al., Reference Rewicz, Grabowski, MacNeil and Bącela-Spychalska2014). Thus, in the long run, P. bosniacus should disperse within the extended range of D. villosus, as has been the case in the Rhine–Main–Danube waterways (David et al., Reference David, Staentzel, Schlumberger, Perrot-Minnot, Beisel and Hardion2018; Reier et al., Reference Reier, Sattmann, Schwaha, Harl, Konecny and Haring2019). This implies, however, that P. bosniacus has not performed any intermediate host captures in the novel area yet.
Host capture
The most interesting finding of this study is the presence of P. tereticollis in G. tigrinus. In North America, this brackish water amphipod serves as the intermediate host of another Pomphorhynchus species, P. rocci (Johnson and Harkema, Reference Johnson and Harkema1971). However, our molecular and morphological data confirmed that the cystacanths harboured by G. tigrinus belong indeed to P. tereticollis and not to P. rocci. While the latter is described as having 15–18 hooks in each longitudinal row, the redescription of P. tereticollis postulates 8–12 hooks per row, fitting our observations (Cordonnier and Ward, Reference Cordonnier and Ward1967; Špakulová et al., Reference Špakulová, Perrot-Minnot and Neuhaus2011).
Considering a significant drop in attention to the parasite communities of the Weser system in the new millennium, it can be assumed that the host capture of G. tigrinus by P. tereticollis took place rather recently, as the characteristically orange larvae would not have remained undiscovered for several decades. On the contrary, no parasitological studies on G. tigrinus in the Weser system were published between 1987 and till date. Therefore, the host capture possibly happened after a period of 40 or more years of the 65 years of co-habitation.
The capture of the non-native host by P. tereticollis might have happened prior to the arrival of Ponto-Caspian invasive species in 1998, when the donor, probably G. pulex, as well as the target host G. tigrinus, temporarily coexisted in the Weser (Grabow et al., Reference Grabow, Eggers and Martens1998). As shown here, the G. pulex population in the Fulda is parasitized by P. laevis, reducing its potential as the donor host for P. tereticollis. It is yet unclear if the host capture is a product of water quality management that resulted in a decrease of salinity (Bäthe and Coring, Reference Bäthe and Coring2011). In contrast, no evidence of a P. tereticollis presence in the upper Werra was discovered; therefore, a return from the unpolluted river sections seems unlikely, though not impossible. Genetic comparison also suggests the cystacanths are closer related to specimen from inland waters rather than of a Baltic Sea origin (Špakulová et al., Reference Špakulová, Perrot-Minnot and Neuhaus2011; Perrot-Minnot et al., Reference Perrot-Minnot, Špakulová, Wattier, Kotlík, Düşen, Aydoğdu and Tougard2018).
Host specificity
As information on the intermediate host specificity of Pomphorhynchus species is scarce, the data presented here add new insights to the puzzling taxonomy of this genus. Contrasts in host patterns should be considered when dealing with parasite taxonomy, as demonstrated by P. cf. minutus. In Germany and France, it was shown to comprise 3 cryptic species, each revealing a high degree of intermediate host specificity (Zittel et al., Reference Zittel, Grabner, Wlecklick, Sures, Leese, Taraschewski and Weigand2018). Polymorphus type 1 only occurred in Gammarus fossarum Koch, 1836 and type 2 in Echinogammarus ischnus (Stebbing, 1899) and Echinogammarus berilloni (Catta, 1878), whereas type 3 (PspT3) was restricted to G. pulex and G. roeselii. Morphological comparison also revealed the latter type to be more different than the other 2, suggesting it might be a non-indigenous form that was introduced with G. roeselii and captured G. pulex as a novel host (Grabner et al., Reference Grabner, Doliwa, Bulantová, Horák and Sures2020). Nevertheless, certain variabilities in the morphological features of the proboscis seem inherent to acanthocephalans (Jirsa et al., Reference Jirsa, Reier and Smales2022). Considering its potential for host adaptation, PspT3 is the most likely candidate to assign the specimen obtained in this study from G. tigrinus. Gammarus roeselii can be found in the unpolluted upstream regions of the Werra and both G. roeselii and G. pulex were found in the Fulda, where also a single G. pulex was infected with P. cf. minutus. Comparison of the single sequence obtained from a Werra specimen against the NCBI database also confirms the assignment to PspT3. As of now, however, due to the lack of more samples, further perspective for the record of P. cf. minutus in G. tigrinus cannot be extrapolated. To our best knowledge, this host–parasite combination has never been mentioned before.
Conclusion
To summarize, the host–parasite associations between acanthocephalans and their amphipod intermediate hosts described here highlight the Weser river system's bio- and especially xenodiversity. Following the jump invasion to an area already colonized by its natural intermediate host G. tigrinus, P. ambiguus did not perform any host captures. In contrast, G. tigrinus was captured as a novel host by the European P. tereticollis within approximately 65 years after the introduction of the American amphipod into the Werra. In contrast, P. bosniacus colonized the Weser together with its invasive host D. villosus and did not show any host captures within the last 2 decades after the arrival of D. villosus in the habitat. Our study exemplifies that in the present times of ecological globalization, communities of hosts and parasites and their associations can be subjected to rapid change and evolution within a few decades.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182023000124.
Data availability
Selected sequences obtained for this study were deposited in NCBI GenBank under the accession numbers ON649686–ON649697 and OQ360657. Additional data not included in this article may be requested from the corresponding author.
Acknowledgements
We acknowledge Heinrich-Böll-Stiftung for financial support of S. V. We thank the group of Bernd Sures at the University Essen-Duisburg, especially Daniel Grabner, for their help in the DNA barcoding process. We also thank Eberhard Frey for providing a workspace for S. V. at the SMNK. We also express our gratitude to Carsten Brauer and Cord Dobberschütz for providing dissection material.
Author's contributions
H. T. conceived and designed the study. S. V. performed sample collection, processing, identification, barcoding and data interpretation. H. T. and S. V. wrote the article. Both authors read and approved the final manuscript.
Financial support
This study is part of the PhD project of S. V. that is financially supported by Heinrich-Böll-Stiftung.
Conflict of interest
The authors declare no conflict of interest.