Obesity is known to be a cause of chronic disease, although the exact mechanisms for this are unclear. Over a decade ago(Reference Hotamisligil, Shargill and Piegelman1), the discovery of a form of subclinical, low-grade systemic inflammation, later called ‘metaflammation’(Reference Hotamisligil2) (referring to metabolically triggered inflammation) associated with both obesity and chronic disease, raised hopes of an improved understanding of the causal links between the two, as this form of inflammation is linked with insulin resistance and chronic disease, and obesity seemed to be the driving cause(Reference Hotamisligil2). However, increasing knowledge about metaflammation, and its connection with a range of lifestyle-related and environmental factors (diet, inactivity, smoking, sleep, stress, pollution, etc), in the absence, as well as in the presence of obesity, suggests an alternative view; that is, obesity may often be just an accomplice to, as much as a perpetrator of, many metabolic diseases. The real (albeit distal) determinants of such diseases appear to lie in aspects of the modern techno-industrial environment enabling and encouraging such lifestyle-related immune stimuli, with an outcome potential in chronic diseases ranging from metabolic disorders(Reference Libby3) to certain forms of cancer(Reference Mantovani, Allovena and Sica4). If this is the case, weight loss, while being a useful component of chronic disease management at the clinical level, may not always be either ‘necessary’ or ‘sufficient’, thus changing the nature of any proposed intervention to manage, at the population level, what should be seen as a natural physiological response to an unnatural, obesogenic(Reference Egger and Swinburn5) environment.
Inflammation and ‘metaflammation’
Classical inflammation represents an acute immune reaction to infection or injury. Metaflammation(Reference Hotamisligil2) differs from this in that: (a) it does not involve the classical symptoms of inflammation (tumour, rubor, dolour and calor); (b) it causes only a small rise in immune system markers (i.e. 4–6-fold v. several 100-fold); (c) it results in chronic, rather than acute, allostasis; (d) it has its effects systemically; (e) its antigens are less apparent as foreign agents or microbial organisms (and hence may be better referred to as ‘inducers’(Reference Medzhitov6)); (f) it appears to perpetuate, rather than resolve, a disease(Reference Hotamisligil, Shargill and Piegelman1–Reference Mantovani, Allovena and Sica4, Reference Medzhitov6). Fig. 1 is a proposed graphical representation of the differences between the two forms of an inflammation.
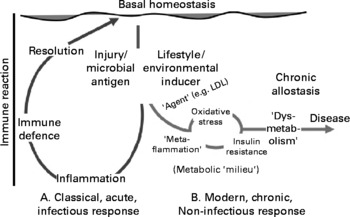
Fig. 1 A graphical representation of the difference between classical inflammation initiated by a microbial antigen or injury and metaflammation caused by lifestyle or environmental inducers. The order and other possible actions in the metabolic ‘milieu’ associated with metaflammation on the right-hand side of the graph are suggestive rather than definitive, but imply the mix of dysmetabolic actions associated with metaflammation. The scale of difference of immune reaction between the two forms (i.e. approximately 100-fold) is not implied. LDL, LDL-cholesterol.
There may be a number of agents associated with inducers of the metaflammatory response (shown on the right-hand side of Fig. 1), oxidised LDL being a known example. As well as aiding the atherogenic process, the leakage of foam cells from the phagocytosis of oxidised LDL formed in the arterial intima, through unstable plaques into the bloodstream, can have a thrombotic impact(Reference Libby3). What is unclear to date, however, is the type and range of initial inducers of this and other processes stimulating metaflammation.
Metaflammatory inducers
Obesity is a cause of oxidative stress and insulin resistance(Reference Libby3) and hence has been proposed as the primary causative factor in chronic and metabolic diseases such as type 2 diabetes(Reference Hajer, van Haeften and Visseren7). However, depending largely on the site of fat storage, obesity can be relatively benign, with little negative impact on health(Reference Frayn8), or metabolic, with apparent links to a range of metabolic and other disorders(Reference Wajchenberg9). Also, a significant proportion of obese individuals suffer none of the dysmetabolism expected from obesity, and a significant proportion of lean individuals do(Reference Karelis, St-Pierre and Conus10, Reference Wildman, Muntner and Reynolds11), suggesting a complicated association between obesity and disease.
There is a known link between obesity and metaflammation(Reference Hotamisligil, Shargill and Piegelman1, Reference Libby3), but the causal relationship is not clear. Metaflammation is also associated with lifestyle and environmental factors that in some instances, but not always, cause obesity(Reference Egger and Dixon12). Awareness of the relationship between postprandial glucose and fat excursions and inflammatory markers(Reference O'Keefe, Gheewala and O'Keefe13), and inflammatory responses to a range of other stimuli(Reference Egger14), has enabled researchers to identify a number of inducers of inflammation (both pro- and anti-) in the body. A list of those with supporting evidence is shown in Table 1.
Table 1 Lifestyle-related inducers with evidence for a pro- (metaflammatory) or anti-inflammatory response in human subjects*
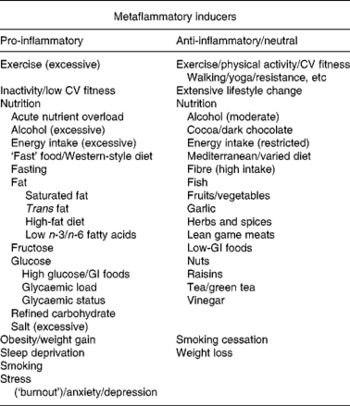
CV, cardiovascular; GI, glycaemic index.
*Stimuli shown on the left-hand side can cause rises in pro-inflammatory markers, often without obesity, whereas those on the right-hand side can cause an opposite reaction, or a reduction of a pro-inflammatory to neutral response (e.g. in the case of smoking cessation and weight loss) often without weight loss. See text and Egger & Dixon(Reference Egger and Dixon12) for detailed references (also at www.lifestylemedicine.net.au/staging/health-information/lifestyle-medicine-evidence-base/inflammation-database/index.htm).
The left-hand side of Table 1 lists inducers with evidence of a pro-inflammatory (metaflammatory) response. As well as obesity and weight gain, this includes excessive alcohol(Reference O'Keefe, Bybee and Lavie15), acute excess energy intake(Reference Solinas, Vilcu and Neels16), a Western-style diet(Reference Esmaillzadeh, Kimiagar and Mehrabi17) and a range of nutritive factors including saturated(Reference Håversen, Danielsson and Fogelstrand18) and trans- fats(Reference Harvey, Arnold and Rasool19), and excessive fructose-(Reference Rayssiguier, Gueux and Nowacki20) and glucose-rich foods(Reference Dickinson, Hancock and Petocz21). Non-nutritive factors include inadequate sleep(Reference Simpson and Dinges22), smoking(Reference Thorley and Tetley23), stress and depression(Reference Kulmatycki and Jamali24). While some of these (e.g. inactivity, excess energy intake) can cause weight gain, this is not a prerequisite for metaflammation to occur. Nutrient overload from acute excessive energy intake, for example, even in the absence of weight gain(Reference Hickling, Hung and Knuiman25), can abnormally tax the intracellular metabolism, cause acute oxidative stress, possibly disrupt normal protein folding in the endoplasmic reticulum(Reference Medzhitov6) and lead to the accumulation of intracellular metabolites, activating inflammatory pathways and inducing insulin resistance(Reference Watt26). Similarly, a high glycaemic index load, or even an excess of otherwise benign low glycaemic index foods, can have an inflammatory effect in the absence of obesity(Reference Galgani, Aguirre and Diaz27). At the other extreme, a similar response results from extended fasting(Reference van der Crabben, Allick and Ackermans28) (probably because of the protective effects of insulin resistance in reducing energy losses). Paradoxically, there are also similar pro-inflammatory effects of both inactivity(Reference Hamburg, McMackin and Huang29) and excessive exercise(Reference Neubauer, König and Wagner30), suggesting a healthy range of certain lifestyle actions, above or below which there is a negative metabolic outcome.
Fig. 2, which has been expanded from a previous article discussing lifestyle-related determinants of inflammation in adolescence(Reference Wärnberg, Nova and Romeo31), distinguishes between those factors with an aetiological effect on inflammation through obesity, and those with an effect ‘independent’ of it.
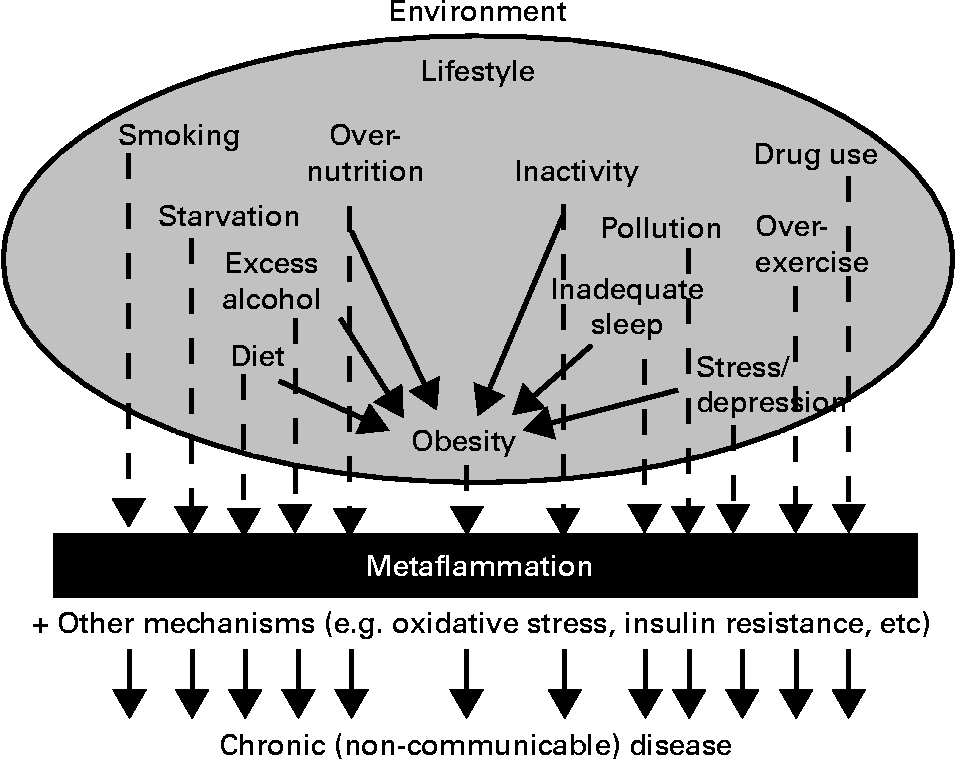
Fig. 2 Environment and lifestyle effects on the development of chronic disease through metaflammation, showing both dependent and independent effects through obesity (expanded from Wärnberg et al.(Reference Wärnberg, Nova and Romeo31)).
Obesity, while often present, may thus not be a necessary condition for metaflammation, or the chronic diseases associated with this. Many of the causes of obesity, on the other hand, as well as other non-obesogenic lifestyle-related inducers, seem to be more directly related aetiologically to chronic disease outcomes through the development of metaflammation. We have discussed this in more detail elsewhere(Reference Egger and Dixon12), pointing out that while the metaflammatory process appears to be a persistent causal factor, the association of inflammation with oxidative stress and insulin resistance in metabolic breakdown (described in Fig. 1 as the metabolic ‘milieu’) is not always clear.
Anti-inflammatory inducers
The pro-inflammatory inducers shown in Table 1 provide some suggestion of a distal causality, as all of these are relatively new to the human environment. This is supported by the identification of the inducers with evidence of an anti-inflammatory effect listed on the right-hand side of Table 1. Anti-inflammatory responses (or neutral responses to corrections of those conditions causing a pro-inflammatory reaction on the left-hand side of Table 1 such as obesity and smoking) have been associated with physical activity and fitness(Reference Brooks, Vasilaki and Larkin32), a healthy lifestyle change(Reference Balagopal, Graham and Kahn33) including smoking cessation(Reference Lao, Jiang and Zhang34), weight loss(Reference You and Nicklas35) and a reduced energy intake(Reference Jolly36), a ‘Mediterranean’ style, or a varied diet(Reference Bulló, Casas-Agustench and Amigó-Correig37), and a range of nutritive factors including a moderate alcohol intake(Reference O'Keefe, Bybee and Lavie15), fish(Reference Nakamura, Ueno and Tamaki38), fruits and vegetables(Reference Wannamethee, Lowe and Rumley39), herbs and spices(Reference Tapsell, Hemphill and Cobiac40), nuts(Reference Kris-Etherton, Hu and Ros41), etc, all of which have been part of the human diet through long periods of evolution. (For a more detailed list of references for both pro- and anti-inflammatory inducers see Egger & Dixon(Reference Egger and Dixon12)).
All these suggest the importance of lifestyle, together with and without obesity, in the dysmetabolism often ascribed to just obesity, as many of these, and other metaflammatory inducers: (a) can have acute effects; (b) can lead to a metaflammatory reaction in the absence of obesity (e.g. smoking, sleep loss, depression); or (c) are not, in themselves, causes of obesity (e.g. smoking, environmental pollution).
Factoring pro- and anti-inflammatory causes
A significant factor distinguishing both sides of Table 1 could be labelled ‘modernity’, associated with the modern techno-industrial environment, arising in the main since around the time of the industrial revolution of the late nineteenth century, with inducers on the left-hand side being largely post-industrial revolution (modern), and thus relatively new to the human repertoire. Those on the right-hand side (anti-inflammatory or neutral), on the other hand, have long been part of the human evolutionary environment. On the assumption that metaflammation is triggered by an immune reaction, this would suggest that while human subjects have psychologically adopted the modern techno-industrial environment with relish, they have not yet successfully adapted to this physiologically.
The fact that these inflammatory environmental inducers and the increase in chronic diseases are linked with modernity, and, in a more distal sense, with economic growth, which is a primary driver of modernity, has been supported by recent epidemiological data, suggesting diminishing returns in aspects of health from economic growth beyond a certain point in advanced economies(Reference Tapia Granados and Ionides42), as well as data from ‘natural’ experiments such as Cuba(Reference Franco, Orduñez and Caballero43) and Japan(Reference Tapia Granados44), in which major economic downturns are paradoxically associated with improvements in chronic disease rates. While not disparaging economic development (in contrast to the pure monetarily measured ‘economic growth’), this supports a view, which is becoming more prevalent(Reference Egger14, Reference Tapia Granados and Ionides42), that investment in growth beyond a certain point begins to yield diminishing returns in health.
Implications for health policy
From the research evidence accumulated over the last decade, cited here and elsewhere(Reference Mantovani, Allovena and Sica4, Reference Medzhitov6, Reference Egger and Dixon12, Reference Egger14), it appears that metaflammation could be the link between a range of lifestyle and environmental factors (including, but not limited to, obesity), and many, if not most, modern chronic diseases. Metaflammation thus becomes to chronic disease what classical inflammation is to injury or microbial invasion, albeit with a different outcome, the latter leading usually to a resolution to homeostasis, but the former leading to dysmetabolism and chronic allostasis (see Fig. 1). Seen in this light, attempts to clinically manage obesity through diets, exercise programmes or medication are unlikely to change chronic disease rates in the community. This by no means negates the importance of weight loss in disease management, as strategies for doing this include changes to the lifestyle causes discussed here. Indeed, a range of simple lifestyle interventions can have a profound effect with the Finnish(Reference Herder, Peltonen and Koenig45), Da Quing(Reference Li, Zhang and Wang46) and US Diabetes Prevention Programs(Reference Haffner, Temprosa and Crandall47), all demonstrating a major reduction in the development of type 2 diabetes in those at high risk, and with greater risk reduction occurring with greater compliance to lifestyle changes, including and independent of weight loss. Recent prospective data from the Nurses Health Study have also shown independent and accumulative reductions in mortality risk over 24 years in US women with increased adherence to modified lifestyle risk factors including and independent of weight loss (not smoking, being physically active and having a healthy diet)(Reference van Dam, Li and Spiegelman48). However, a population approach to chronic disease reduction calls for something more than volitional behaviour change in one or two areas of behaviour associated with weight loss.
Obesity is a normal response to an abnormal environment(Reference Egger and Swinburn5). Such an environment has been called obesogenic(Reference Egger and Swinburn5), and, more recently, inflammatory(Reference Egger14), metaphorically linking obesity with climate change. Increased levels of obesity in the community in this light can be seen as ‘… the unintended but unavoidable consequences of economic progress’(Reference Roberts49), presenting as an accomplice, as much as a perpetrator, in many, if not all, of the chronic diseases associated with that modernity.
The hypothesis proposed here – that human subjects have an induced inflammatory reaction to the modern techno-industrial environment to which they have not evolved or adequately adapted – poses some interesting questions and provides challenging dilemmas and possible solutions in relation to this.
First, how can the distal determinants of such a response (e.g. industrialisation/the modern Western environment/economic growth) be managed without abandoning the obvious human advantages they have brought to date? There is little doubt that, to a point at least(Reference Tapia Granados and Ionides42), economic growth has been the single biggest driving force behind improved health and well-being since the start of the industrial revolution(Reference Riley50). The world economic crisis beginning in 2007–8 has signalled to many, however, not just a need for a reordering of the current system, but for a paradigm shift in the growth system of economics(Reference Daly51, Reference Meadows, Randers and Meadows52), thus echoing the views of its early architects such as Mill(Reference Mill53) and Keynes(Reference Keynes54) that growth would need to be pursued for a time, but eventually would need to be replaced by an alternative system that focused more on human improvement(Reference Mill53) (presumably including health). This is because, as recognised then, but often overlooked in the current growth-dominated political environment, nothing can grow forever. As stated by one observer: ‘… after maturity, continued growth is either obesity or cancer’(Reference Bartlett55). This also signals the need for health scientists to become involved in the economic debate.
Second, what is the connection between obesity and chronic disease in the absence of damaging lifestyle cofactors? Does the fit-but-fat phenomenon reduce the need for a population emphasis on weight control in favour of an increased emphasis on other aspects of lifestyle (such as activity levels, sleep, stress management, etc) and the inflammatory environment, with possible beneficial impact on both the biological and ecological environments(Reference Egger14)? Health and environmental issues, such as pollution and climate change, have recently been shown to be intimately associated through the use of non-renewable fossil fuels in transport, also leading to reduced personal energy expenditure and increased obesity(Reference Egger14, Reference Faergeman56).
Finally, what can be done to counteract this environment-initiated epidemic, given that a return to a pre-industrial society is unlikely? It is not the intention here to detail what will obviously be a complex and multidisciplinary response, which is often considered outside the realm of health scientists, but which we propose is integral to the management of chronic disease. There is a need for an array of workable solutions at many levels of modern society, and away from an isolated view of health and obesity as simply a pharmaceutical problem. While a major economic paradigm shift such as that suggested previously is unlikely to happen in the immediate future, interim initiatives such as corporate and personal carbon trading have begun to be put in place to moderate the effects of unlimited growth, albeit serendipitously through rising interest in climate change(Reference Egger14, Reference Faergeman56), but also with a potential benefit in managing the chronic disease epidemic(Reference Egger and Dixon12, Reference Egger14, Reference Egger57). There are still many answers to be provided, however, and undoubtedly many more questions arising from these suggestions, for which health scientists will need to expand their horizons.
Acknowledgements
The authors acknowledge no conflicts of interest or funding for the present paper. G. E. acted as the main author and J. D. contributed as the joint author to the final copy.





