The importance of arachidonic acid (20 : 4n-6, ARA), an n-6 long-chain PUFA, in fish nutrition has tended to be overlooked compared with EPA (20 : 5n-3) and DHA (22 : 6n-3) in the early research, because the latter two long-chain PUFA are more dominant than ARA in fish tissues( Reference Koven, Barr and Lutzky 1 , Reference Bell and Sargent 2 ) and because the contribution of ARA to growth and survival is easily masked if other essential fatty acid levels are suboptimal( Reference Xu, Ai and Mai 3 ). However, in recent decades, it has been shown that ARA can be metabolised to form highly bioactive eicosanoids, such as PG, thromboxanes and leukotrienes (LT), which are very active even at low physiological concentrations and play critical roles in the regulation of several biological processes( Reference Needleman, Jakschik and Morrison 4 , Reference Zeldin 5 ). The potential benefits of optimal ARA nutrition to fish physiology and biochemistry have gradually become recognised( Reference Bell and Sargent 2 ), and a number of studies have been conducted to investigate the effects of dietary ARA in various fish species( Reference Xu, Ai and Mai 3 , Reference Bransden, Cobcroft and Battaglene 6 – Reference Willey, Bengtson and Harel 13 ).
Previous studies have demonstrated that ARA is preferentially retained together with DHA during starvation in many fish species, which suggests that it has the same metabolic priority for conservation and relative importance as DHA( Reference Rainuzzo, Reitan and Jorgensen 14 , Reference Izquierdo 15 ). Several studies have also revealed that dietary ARA contributes to enhanced survival and growth rates( Reference Bessonart, Izquierdo and Salhi 7 , Reference Lund, Steenfeldt and Hansen 9 , Reference Luo, Tan and Li 10 , Reference Rezek, Watanabe and Harel 16 ) and influences the tissue fatty acid profile( Reference Villalta, Estévez and Bransden 12 , Reference Willey, Bengtson and Harel 13 , Reference Atalah, Hernández-Cruz and Benítez-Santana 17 ) in a variety of teleosts. In particular, ARA has been reported to play an important role in regulating the reproductive performance of brood stock( Reference Kobayashi, Sorensen and Stacey 18 – Reference Ohs, DiMaggio and Grabe 22 ) and the stress resistance of larval fish( Reference Koven, Barr and Lutzky 1 , Reference Bransden, Cobcroft and Battaglene 6 , Reference Carrier, Watanabe and Harel 8 , Reference Martins, Rocha and Castanheira 11 , Reference Van Anholt, Koven and Lutzky 23 – Reference Atalah, Hernández-Cruz and Ganuza 25 ). Moreover, the effects of ARA on a range of physiological processes in marine fish, such as metamorphosis( Reference Copeman, Parrish and Brown 26 , Reference Lund, Steenfeldt and Banta 27 ), pigmentation( Reference Lund, Steenfeldt and Hansen 9 , Reference Estevez, Ishikawa and Kanazawa 28 , Reference Bransden, Butterfield and Walden 29 ) and immune response( Reference Xu, Ai and Mai 3 , Reference Li, Ai and Mai 30 ), have also been investigated by different research groups. However, no study to date has focused on the metabolic mechanism of ARA in marine fish.
In mammals, the metabolism of ARA and the function of ARA metabolites have been widely investigated( Reference Astudillo, Balgoma and Balboa 31 ). Membrane-bound endogenous fatty acid ARA can be released from membranes by phospholipases and then metabolised to biologically active compounds by cyclo-oxygenases (COX), lipoxygenases (LOX) and cytochrome P450 (CYP) enzymes( Reference Needleman, Jakschik and Morrison 4 , Reference Astudillo, Balgoma and Balboa 31 , Reference Rifkind, Lee and Chang 32 ). ARA is mainly metabolised to three distinct classes of metabolites in animal models and humans: COX produces PG and thromboxanes; LOX produces hydroperoxyeicosatetraenoic acids, LT and lipoxins; and the CYP enzyme, an NADPH-dependent epoxygenase, produces epoxyeicosatrienoic acids (EET)( Reference Astudillo, Balgoma and Balboa 31 ). These metabolites have been shown to play important functional roles in a variety of fundamental biological processes, such as cellular proliferation, inflammation, vascular and bronchial smooth muscle tone, peptide hormone secretion and intracellular signalling( Reference Zeldin 5 , Reference Samuelsson 33 – Reference Brash 35 ). In fish species, investigations involved in ARA metabolism have generally focused on the COX (especially the COX-2) pathway( Reference Martins, Rocha and Castanheira 11 , Reference Van Anholt, Koven and Lutzky 23 , Reference Oxley, Jolly and Eide 36 ), and few studies have been conducted on the other metabolic pathways of ARA.
COX-1 and COX-2 are the key enzymes responsible for prostanoid production from ARA( Reference Langenbach, Morham and Tiano 37 ). Under many circumstances, the COX-1 enzyme is produced constitutively, whereas COX-2 is inducible( Reference Dubois, Abramson and Crofford 38 ). The constitutively expressed COX-1 is responsible for basal synthesis and, upon stimulation, for immediate PG synthesis, which also occurs at high ARA concentrations( Reference Legler, Bruckner and Uetz-von Allmen 39 ). COX-2 is induced by a variety of stimuli, such as cytokines and growth factors, and it is primarily involved in the regulation of inflammatory responses as well as cell differentiation and proliferation( Reference Legler, Bruckner and Uetz-von Allmen 39 , Reference Vane, Bakhle and Botting 40 ). 5-LOX is a member of the family of LOX that also includes 12- and 15-LOX( Reference Ford-Hutchinson, Gresser and Young 41 ). It catalyses ARA to form 5-hydroperoxyeicosatetraenoic acid and subsequently metabolises to LT( Reference Needleman, Jakschik and Morrison 4 , Reference Ford-Hutchinson, Gresser and Young 41 ). 5-LOX is the rate-limiting enzyme in LT synthesis( Reference Mehrabian, Allayee and Wong 42 ). CYP enzymes comprise a highly diverse superfamily found in all domains of life, and one of the important physiological roles of CYP enzymes is that they are involved in the metabolism of ARA( Reference Kirischian, McArthur and Jesuthasan 43 ). CYP monooxygenases catalyse the epoxidation of ARA to form EET( Reference Zeldin, Foley and Ma 44 , Reference Capdevila, Falck and Harris 45 ). EET are endogenous constituents of numerous tissues and possess a variety of potent biological activities, such as controlling peptide hormone secretion in the pancreas, pituitary gland and hypothalamus and regulating vascular tone in the intestine and brain( Reference Wang, Yao and Chen 46 ). In vertebrates, the CYP4A, CYP2C and CYP2J subfamilies were generally believed to be the major enzymes involved in EET synthesis from ARA( Reference Capdevila, Falck and Imig 47 , Reference Zordoky and El-Kadi 48 ). However, no study has reported the effects of dietary ARA concentrations on the gene expression of COX-1, 5-LOX and CYP enzymes in marine fish, and the effect of different dietary ARA concentrations on COX-2 mRNA expression is also rarely reported( Reference Martins, Rocha and Castanheira 11 ).
Half-smooth tongue sole (Cynoglossus semilaevis) is a high-value marine flatfish with a notably lethargic browsing feeding habit( Reference Ma, Liu and Xu 50 ), and it is extensively exploited in northern China( Reference Liu, Xu and Ma 49 ). A few studies have been reported on the nutrition of tongue sole( Reference Ma, Liu and Xu 50 – Reference Tian, Fang and Dong 53 ), but no information is available about its ARA nutrition. ARA can be converted to a number of compounds, including PG, thromboxanes, hydroxyeicosatetraenoic acids and LT( Reference Astudillo, Balgoma and Balboa 31 ), which are very active even at low physiological concentrations and play an important role during larval development( Reference Bessonart, Izquierdo and Salhi 7 ). It has been demonstrated that bone development and composition at the larval stage are highly sensitive to the dietary concentration of ARA( Reference Boglino, Darias and Andree 54 ). In addition, fish larvae possess a high growth rate( Reference Houde 55 ), which indicates that metabolism in this stage is active; thus, tongue sole larvae appear to be more sensitive to ARA nutrition than juvenile tongue sole are.
Hence, the present study was designed to determine the effects of dietary ARA concentrations on growth performance, the activities of digestive enzymes and the fatty acid profiles of larval tongue sole. The responses of some ARA metabolism-related gene expression to dietary ARA concentrations were also investigated.
Materials and methods
Feed ingredients and diet formulation
A total of six diets were formulated to contain approximately 56 % crude protein and 14 % lipids, a combination that has been shown to be sufficient to support the optimal growth of larval tongue sole (Table 1). ARA-enriched oil (ARA content: 53·78 % of total fatty acid in the form of ARA-methylester; Jiangsu Tiankai Biotechnology Company Limited) was supplemented to the basal diet separately as a substitute for palmitin (palmitic acid content: 97·6 % of total fatty acid in the form of methylester; Shanghai Zhixin Chemical Company Limited) in order to create six diets that contained ARA levels of 0·01, 0·39, 0·70, 1·07, 1·42 and 2·86 % dry weight, respectively. DHA-enriched oil (DHA content: 40·64 % of total fatty acid in the form of DHA-methylester; Jiangsu Tiankai Biotechnology Company Limited) and EPA-enriched oil (45·9 % EPA and 23·8 % DHA of total fatty acid, both in the form of TAG; Hebei Haiyuan Health Biological Science and Technology Company Limited) were added to keep the DHA:EPA ratio at approximately 2·0 (Table 2). Defatted fishmeal, krill meal, squid meal and hydrolysed fishmeal, together with casein, were chosen as the primary protein sources.
Table 1 Formulation and proximate analysis of the experimental diets (% dry weight)

ARA, arachidonic acid.
* Defatted fishmeal: crude protein 77·81 % DM, crude lipid 2·88 % DM (white fishmeal were defatted with ethanol (fishmeal–ethanol 1:2, w:v) at 37°C three times); casein: crude protein 92·34 % DM, crude lipid 0·89 % DM (all supplied by Qingdao Great Seven Bio-Tech Company Limited).
† Krill meal: crude protein 52·99 % DM, crude lipid 12·95 % DM (Shandong Keruier Biological Products Company Limited); squid meal: crude protein 62·72 % DM, crude lipid 3·5 % DM; hydrolysed fishmeal: crude protein 75·98 % DM, crude lipid 1·34 % DM (Zhejiang Jinhaiyun Biology Company Limited).
‡ DHA-enriched oil: DHA content 40·64 % of total fatty acid (TFA) in the form of DHA-methylester (Jiangsu Tiankai Biotechnology Company Limited); EPA-enriched oil: EPA content 45·9 % of TFA, DHA content 23·8 % of TFA, both in the form of TAG (Hebei Haiyuan Health Biological Science and Technology Company Limited); ARA-enriched oil: ARA content 53·78 % of TFA in the form of ARA-methylester (Jiangsu Tiankai Biotechnology Company Limited); palmitin: palmitic acid (16 : 0) content 97·60 % of TFA in the form of methylester (Shanghai Zhixin Chemical Company Limited).
§ Vitamin premix (IU or g/kg vitamin premix): retinal palmitate, 3 000 000 IU; cholecalciferol, 1 200 000 IU; dl-α-tocopherol acetate, 40·0 g; menadione, 8·0 g; thiamin–HCl, 5·0 g; riboflavin, 5·0 g; d-calcium pantothenate, 16·0 g; pyridoxine–HCl, 4·0 g; meso-inositol, 200·0 g; d-biotin, 8·0 g; folic acid, 1·5 g; para-aminobenzoic acid, 5·0 g; niacin, 20·0 g; cyanocobalamin, 0·01 g; ascorbyl polyphosphate (containing 25 % ascorbic acid), 100·0 g.
∥ Mineral premix (g/kg): Ca(H2PO4)2.H2O, 675·0; CoSO4·4H2O, 0·15; CuSO4·5H2O, 5·0; FeSO4·7H2O, 50·0; KCl, 50·0; KI, 0·1; MgSO4·2H2O, 101·7; MnSO4·4H2O, 18·0; NaCl, 80·0; Na2SeO3.H2O, 0·05; ZnSO4·7H2O, 20·0.
¶ Attractant (g/100 g): betaine, 50; glycine, 15; alanine, 10; argine, 10; taurine, 10; inosine-5′-monophosphoric acid, 5.
** Antioxidant: ethoxyquin.
†† Mould inhibitor: 50 % calcium propionic acid, 50 % fumaric acid.
Table 2 Fatty acid composition of the experimental diets (% total fatty acids)*

ARA, arachidonic acid.
* Some fatty acids, of which the contents were minor, in trace amounts or not detected (such as 22 : 0, 24 : 0, 14 : 1, 20 : 1n-9, 22 : 1n-11, 20 : 2n-6, 20 : 3n-6 and 22 : 5n-3), are not listed in Table 2.
The primary ingredients were ground into a fine powder through 75 μm mesh. After that, the ingredients for all of the diets were blended manually, and then the oil mixtures (ARA-enriched oil, DHA-enriched oil, EPA-enriched oil, palmitin and lecithin) were added to each diet and mixed thoroughly with the other ingredients. Water was incorporated to produce a stiff dough. Pellets were created with an automatic pellet-making machine (Weihai) and dried for about 8 h in a ventilated oven at 45°C. After drying, the pellets were broken and sieved to obtain two particle sizes: 180–250 μm for the fish larvae between 35 and 50 d after hatching and 250–420 μm for the larval fish between 50 and 65 d after hatching. All of the formulated diets were packed in separate silver bags and stored at − 20°C until they were used.
Experimental procedure
The larvae used in the present study were obtained and reared at the hatchery of the Haiyang Seafood Company in Yantai (Shandong, China). Before the experiment, the initial wet body weights of 100 randomly sampled larvae were measured. With a stocking destiny of 180 individuals per tank, a total of 3240 larvae (35 d after hatching, 54 (sem 1) mg) were randomly distributed into eighteen tanks with flat bottoms (65 × 65 × 90 cm). Seawater was continuously pumped from the coast adjacent to the experiment station and passed through sand filters into each tank. About 200–400 % of the water volume was renewed daily, and each tank had an air stone. The feeding trial lasted for 30 d. At the beginning, the fish were fasted for 24 h, and each diet was randomly assigned to three groups of fish. Enriched Artemia nauplii and a micro-diet were used alternately to wean the larvae for 3 d before the formal experiment began. Fish were manually fed with the corresponding experimental diets to apparent satiation five times daily (at 06.00, 9.00, 15.00, 18.00 and 21.00 hours). During the rearing period, water temperature was kept at 24 ± 1°C, pH was 8·0 ± 0·2 and salinity was 30 ± 3 ‰. The surface water was skimmed with a polyvinyl chloride (PVC) tube to remove the suspended waste. Also, accumulations of feed and faeces at the tank bottoms were siphoned 40 min after feeding. At the termination of the experiment, thirty fish were randomly sampled from each tank to determine wet body weight. All of the fish were deprived of food for 24 h before sampling. Survival was determined by counting the individuals remaining in each tank. All of the larvae were anaesthetised with eugenol (1:10 000) (Shanghai Reagent) and rinsed in distilled water before further treatment; larvae that were collected for further assays were immediately frozen in liquid N2 and then stored at − 80°C.
Biochemical analysis
The chemical composition of the diets was determined according to the standard procedures( 56 ). Samples of the diets were dried to a constant weight at 105°C to determine their DM content. Crude protein was determined by digestion using the Kjeldahl method (N × 6·25); crude lipid was measured by diethyl ether extraction using the Soxhlet method.
The fatty acid profiles were analysed using the procedure described by Metcalfe et al. ( Reference Metcalfe, Schmitz and Pelka 57 ) with some modification( Reference Ai, Zhao and Mai 58 ). Fatty acid methyl esters were separated and quantified using HP6890 gas chromatograph equipment (Agilents Technologies, Inc.) with a fused silica capillary column (007-CW; Hewlett Packard) and a flame ionisation detector. The column temperature was programmed to rise from 150 to 200°C at a rate of 15°C/min, from 200 to 270°C at a rate of 2°C/min and then kept steady for 10 min. The injector and detector temperature was 270°C. A Supelco 37 Component FAME Mix (NU-CHEK) and an HPCore ChemStation workstation were used to identify and quantify each fatty acid after separation using GC.
Digestive enzymatic assays
Enzymatic assays were determined on the pancreatic segments (PS), intestinal segments (IS) and brush border membranes (BBM) of the digestive tracts. The larvae were dissected on a glass plate maintained at 0°C according to the methods for sole described previously by Ribeiro et al. ( Reference Ribeiro, Zambonino-Infante and Cahu 59 ). Digestive tracts were separated at the junction of the oesophagus and the pyloric sphincter to obtain the PS and IS. Dissected samples were refrozen at − 80°C for the enzymatic assays. Purified BBM from the IS homogenate was obtained following the method developed for intestinal scrapings by Crane et al. ( Reference Crane, Boge and Rigal 60 ) and Zambonino Infante et al. ( Reference Zambonino Infante, Cahu and Péres 61 ). Trypsin activity and amylase activity were assayed using Na-benzoyl-dl-arginine-p-nitroanilide (B-4875)( Reference Holm, Hanssen and Krogdahl 62 ) and starch (S-9765)( Reference Métais and Bieth 63 ) as substrates, respectively. In both the PS and the IS, lipase activities were assayed using polyvinyl alcohol (PVA)-olive oil emulsion as the substrate according to the method described by Brockman( Reference Brockman 64 ). Alkaline phosphatase (ALP) activity was measured using p-nitrophenylphosphate (106850; Merck) and MgCl2 as the substrates( Reference Bessey, Lowry and Brock 65 ), and leucine aminopeptidase (LAP) activity was measured using leucine-p-nitroanilide (L-9125; Sigma) as the substrate( Reference Appel 66 ). Protein concentration was determined according to the method described by Bradford( Reference Bradford 67 ) using bovine serum albumin (A-2153; Sigma) as a standard. Enzyme activities are expressed as specific activity (mU mg/protein or U mg/protein).
A single unit of enzyme activity was defined as the amount of enzyme that hydrolyses 1 μmol of substrate per min at 37°C for ALP and LAP and at 25°C for trypsin. Amylase activity was expressed as the equivalent enzyme activity required to hydrolyse 1 mg of starch in 30 min at 37°C. A single unit of lipase activity was defined as 1 μmol of fatty acid released by the hydrolysing lipid in 1 min at 37°C.
RNA extraction and reverse transcription
Total RNA was extracted from larval visceral mass using Trizol Reagent (Invitrogen) according to the manufacturer's instructions and electrophoresed on a 1·2 % denaturing agarose gel to test the integrity. The purity and concentration of total RNA were determined by NanoDrop® ND-1000. The absorption ratios (260:280 nm) for all of the samples were approximately 2·00. Then first-strand complementary DNA (cDNA) was synthesised using a PrimeScript™ RT Reagent Kit (Takara) according to the manufacturer's instructions. The resulting product was used as a template for PCR amplification.
Partial cloning of arachidonic acid metabolism-related genes
In order to obtain fragments of the COX-1, COX-2, 5-LOX and CYP2J genes, four pairs of degenerate PCR primers (Table 3) were designed by Primer Premier 5.0 in highly conserved regions on the basis of available sequences in Genbank and were synthesised by Biosune Biotech. PCR was performed on a volume of 25 μl that contained 1 μl each of primer (10 μm) and cDNA, 9·5 μl of sterilised double-distilled water and 12·5 μl of Taq Mix (TransGen Biotech). The PCR programme was carried out by Eppendorf Mastercycler Gradient (Eppendorf), and the PCR conditions were: 2 min at 94°C; thirty-five cycles of 30 s at 94°C, 30 s at the annealing temperature (Table 3) and 40 s (changed according to the length of the target gene) at 72°C; and then another 10 min at 72°C. The amplification products were separated by electrophoresis on a 1·5 % agarose gel according to their length, and then the target band was ligated into the pEASY-T1 vector (TransGen Biotech). A total of 2 μl of each ligation reaction were transformed into the competent cells of Escherichia coli TOP10 and then plated on Lysogeny broth (LB) agar plates. The positive clones in each PCR fragment were sequenced in Sangon Biotech. Sequence alignment and analysis were conducted using the BLAST sequence analysis service of the National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov/). Multiple alignments of the target genes were performed with the ClustalW Multiple Alignment Program (http://www.ebi.ac.uk/Tools/msa/clustalo/).
Table 3 PCR primers for arachidonic acid metabolism-related gene cloning of larval tongue sole (Cynoglossus semilaevis)

COX-1, cyclo-oxygenase 1; COX-2, cyclo-oxygenase 2; 5-LOX, 5-lipoxygenase; CYP2J, cytochrome P450 2J.
Real-time quantitative PCR
The quantitative PCR primer pairs (Table 4) were designed by Primer Premier 5.0 based on the obtained nucleotide sequences of COX-1a (GenBank accession no. KF533723), COX-1b (GenBank accession no. KF533724), COX-2 (GenBank accession no. KF533725), 5-LOX (GenBank accession no. KF533726), CYP2J6-like (GenBank accession no. KJ578726) and β-2 microglobulin (GenBank accession no. FJ965561) in tongue sole. The mRNA expression levels were normalised to β-2 microglobulin. Real-time quantitative PCR was carried out in a quantitative thermal cycler (Mastercycler ep realplex; Eppendorf). The amplification was performed on a total volume of 25 μl that contained 1 μl of primer (10 μm), 1 μl of the diluted first-strand cDNA product, 12·5 μl of 2 × SYBR® Premix Ex Taq™ and 9·5 μl of sterilised double-distilled water. The quantitative PCR programme was as follows: 95°C for 2 min, followed by forty cycles of 10 s at 95°C, 10 s at 58°C and 20 s at 72°C. At the end of each PCR, melting curve analysis was performed to confirm that only one PCR product was present in these reactions. Standard curves were made with six different dilutions (in triplicate) of the cDNA samples, and amplification efficiency was analysed according to the following equation: E= 10( − 1/slope)− 1(
Reference Livak and Schmittgen
68
). The primers' amplification efficiencies were 0·940 (R
2 0·998), 0·960 (R
2 0·991), 0·978 (R
2 0·998), 0·951 (R
2 0·987), 0·954 (R
2 0·998) and 0·948 (R
2 0·995) for COX-1a, COX-1b, COX-2, 5-LOX, CYP2J6-like and β-2 microglobulin, respectively. Plots of the log DNA dilution v. ΔC
t (C
t, target gene− C
t, β-2microglobulin) were made, and the slopes were calculated. The absolute values of the slopes were all close to zero, which indicates that the efficiencies of the target and reference genes were similar, and the expression levels of the target genes were calculated following the
![]() $$2^{ - \Delta \Delta C _{ t }} $$
method described by Livak & Schmittgen(
Reference Livak and Schmittgen
68
).
$$2^{ - \Delta \Delta C _{ t }} $$
method described by Livak & Schmittgen(
Reference Livak and Schmittgen
68
).
Table 4 Real-time quantitative PCR primers for arachidonic acid metabolism-related genes and β-2 microglobulin of larval tongue sole (Cynoglossus semilaevis)

COX-1a, cyclo-oxygenase 1a; COX-1b, cyclo-oxygenase 1b; COX-2, cyclo-oxygenase 2; 5-LOX, 5-lipoxygenase; CYP2J6-like, cytochrome P450 2J6-like.
Calculations and statistical analysis
The following variables were calculated:
where Σ(t× feeding days) was the sum of the water temperatures (°C) for every feeding day in the experiment.
where N fi and N ft were the initial and final fish number in the experiment, respectively.
All of the statistical evaluations were performed using SPSS version 19.0 (SPSS, Inc.). Polynomial contrasts (linear, quadratic and cubic) were used to test the effect of the dietary ARA concentrations on the various variables measured. The level of significance was set at P< 0·05, and the results are presented as mean values with pooled standard errors.
Results
Survival and growth
An increase in dietary ARA concentration caused a non-linear rise to a plateau in SR, final body weight and TGC. When the dietary ARA concentration increased from 0·01 to 1·42 %, SR increased from 20·91 to 30·53 %, and larvae that were fed the diet with 1·42 % ARA had the highest SR as compared to the other treatments. Also, in the 1·42 % dietary ARA treatment, final body weight obtained the highest value as compared to the other treatments. The TGC of larvae fed the diet with 1·42 % ARA was much higher than that in the other treatments, and the 0·01 % ARA treatment showed the lowest TGC (Table 5).
Table 5 Growth response and survival rates of larval tongue sole fed diets with graded concentrations of arachidonic acid (ARA) (Mean values with their pooled standard errors)*
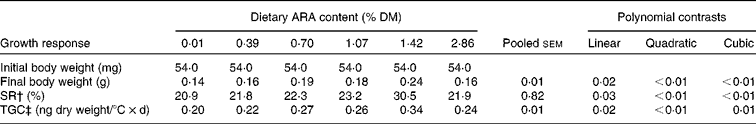
SR, survival ratio; TGC, thermal growth coefficient.
* Values are means of triplicate.
† SR (%) = 100 × final fish number/initial fish number.
‡ TGC (ng dry weight/°C × d) = (W f 1/3− W i 1/3) × 1000/Σ(t× feeding days), where W f and W i were the final and initial fish weights, respectively, and Σ(t× feeding days) was the sum of the water temperatures (°C) for every feeding day in the experiment.
Activity of digestive enzymes
The activity of trypsin in larval PS increased significantly in a ‘U’-shaped manner as dietary ARA increased from 0·01 to 1·07 % and decreased unsymmetrically at higher ARA concentrations. Larvae fed the diet with 2·86 % ARA showed the lowest trypsin activity in the PS. In the IS, the lowest trypsin activity was seen in the 0·01 % dietary ARA treatment, and trypsin activity increased significantly in a non-linear manner to a plateau as dietary ARA increased from 0·01 to 1·42 %; it then decreased unsymmetrically. Lipase activity in both the PS and IS increased significantly in a ‘U’-shaped manner as dietary ARA increased from 0·01 to 1·42 % and then decreased symmetrically, but the values of lipase activity in most of the treatments were close to each other. The highest amylase activity in the PS and IS occurred in the 0·01 and 0·39 % ARA treatments, respectively. LAP activity in both the IS and BBM increased significantly to a plateau in a non-linear manner as dietary ARA increased from 0·01 to 1·42 %, and then it decreased. ALP activity in the IS followed the same pattern as LAP. However, ALP activity in the BBM increased significantly in a ‘U’-shaped manner as dietary ARA increased from 0·01 to 1·42 %, and then it decreased symmetrically. The lowest ALP activity in the IS and BBM was seen in the 0·01 and 2·86 % ARA treatments, respectively (Table 6).
Table 6 Digestive enzyme activity of larval tongue sole fed diets with graded concentrations of arachidonic acid (ARA) (Mean values with their pooled standard errors)*

Prot, protein; PS, pancreatic segment; IS, intestinal segment; LAP, leucine aminopeptidase; BBM, brush-border-membrane; ALP, alkaline phosphatise.
* Values are means of triplicate.
Fatty acid composition
The fatty acid composition of the fish larvae is presented in Table 7. SFA, n-6 PUFA and EPA in the larvae were much lower than in the diets, but MUFA and DHA in the larvae were higher than in the diets. Meanwhile, n-3:n-6 PUFA and DHA:EPA in the larvae were also higher than in the diets. As dietary ARA concentration increased from 0·01 to 1·42 %, SFA and EPA decreased significantly in a linear manner, and DHA increased in a ‘U’-shaped manner and then decreased unsymmetrically. This also led to a changing ratio of DHA to EPA in fish larvae, and the larvae fed 1·42 % dietary ARA had much higher DHA:EPA as compared to other treatments. Larvae fed 2·86 % dietary ARA showed the lowest SFA and DHA. On the contrary, the amount of MUFA, especially 18 : 1n-7, in larvae fed 2·86 % dietary ARA was much higher than that in other treatments. The ARA in fish larvae increased significantly to a plateau in a non-linear manner as dietary ARA concentration increased, and the larvae fed 0·70 % dietary ARA had higher n-6 PUFA.
Table 7 Whole-body fatty acid composition of larval tongue sole fed diets with graded concentrations of arachidonic acid (ARA, % total fatty acid) (Mean values with their pooled standard errors)*

* Some fatty acids, of which the contents were minor, in trace amounts or not detected (such as 22 : 0, 24 : 0, 14 : 1, 20 : 1n-9, 22 : 1n-11, 20 : 2n-6, 20 : 3n-6 and 22 : 5n-3), are not listed in Table 7. Data are means of triplicate.
Partial nucleotide sequences of arachidonic acid metabolism-related genes
The present study obtained the partial nucleotide sequences of COX-1a, COX-1b, COX-2, 5-LOX and CYP2J6-like cDNA, and the nucleotide sequence data of these genes have been deposited in the GenBank nucleotide sequence database under accession no. KF533723, KF533724, KF533725, KF533726 and KJ578726, respectively. The sequence of COX-1a revealed a fragment of 788 bp that was highly homologous to Myoxocephalus octodecemspinosus (81 %) and Fundulus heteroclitus (81 %), and the sequence of COX-1b revealed a fragment of 772 bp that was also highly homologous to M. octodecemspinosus (81 %) and F. heteroclitus (81 %). The sequence of COX-2 revealed a fragment of 1037 bp that was highly homologous to Oplegnathus fasciatus (82 %), Micropogonias undulates (81 %) and Pagrus major (80 %). The sequence of 5-LOX revealed a fragment of 1014 bp that was highly homologous to Oreochromis niloticus (81 %), Maylandia zebra (80 %) and Takifugu rubripes (79 %). The sequence of CYP2J6-like revealed a fragment of 871 bp that was highly homologous to Pundamilia nyererei (76 %), Haplochromis burtoni (76 %) and M. zebra (76 %).
Arachidonic acid metabolism-related gene expression
The relative mRNA expression levels of ARA metabolism-related genes (COX-1a, COX-1b, COX-2, 5-LOX and CYP2J6-like) in the visceral mass of larvae that were fed diets with graded concentrations of ARA is presented in Figs. 1 and 2. The relative expression of COX-1a and COX-1b showed similar changing trends with increased dietary ARA, but few differences were found in the relative expression of both COX-1a and COX-1b among the dietary treatments. Both the COX-2 and 5-LOX mRNA expression levels significantly increased to a plateau in an ‘L’-shaped manner, and the maximum expression levels of COX-2 and 5-LOX were seen in the 1·42 and 1·07 % dietary ARA treatments, respectively. The expression level of COX-2 increased approximately 0·68- and 0·25-fold in the treatments with 1·42 and 2·86 % dietary ARA, respectively (Fig. 2(a)), and the expression levels of 5-LOX were increased about 0·61- and 0·60-fold in the treatments with 1·07 and 1·42 % dietary ARA, respectively (Fig. 2(b)). Although the expression levels of CYP2J6-like transcript were up-regulated to a maximum as dietary ARA increased from 0·01 to 1·42 % and were then down-regulated, little difference was observed (Fig. 2(c)).

Fig. 1 Relative mRNA expression of cyclo-oxygenase (COX)-1a and COX-1b in the visceral mass of larval tongue sole (Cynoglossus semilaevis) fed with graded concentrations of arachidonic acid (ARA). Relative mRNA expression was evaluated by real-time quantitative PCR. Values are means (n 3), with their standard errors represented by vertical bars. COX-1a (□): P
linear= 0·82; P
quadratic= 0·92; P
cubic= 0·18. COX-1b (![]() ): P
linear= 0·55; P
quadratic= 0·08; P
cubic= 0·06.
): P
linear= 0·55; P
quadratic= 0·08; P
cubic= 0·06.

Fig. 2 Relative mRNA expression of cyclo-oxygenase (COX)-2 (a), 5-lipoxygenase (5-LOX) (b) and cytochrome P450 2J6-like (CYP2J6-like) (c) in the visceral mass of larval tongue sole (Cynoglossus semilaevis) fed with graded concentrations of arachidonic acid (ARA). Relative mRNA expression was evaluated by real-time quantitative PCR. Values are means (n 3), with their standard errors represented by vertical bars. COX-2 (![]() ): P
linear= 0·04; P
quadratic= 0·33; P
cubic< 0·01. 5-LOX (
): P
linear= 0·04; P
quadratic= 0·33; P
cubic< 0·01. 5-LOX (![]() ): P
linear= 0·35; P
quadratic< 0·01; P
cubic= 0·02. CYP2J6-like (
): P
linear= 0·35; P
quadratic< 0·01; P
cubic= 0·02. CYP2J6-like (![]() ): P
linear= 0·14; P
quadratic= 0·80; P
cubic= 0·20.
): P
linear= 0·14; P
quadratic= 0·80; P
cubic= 0·20.
Discussion
The results of the present study demonstrated that the growth and survival of larval tongue sole responded to dietary ARA with a non-linear raise to a plateau, which implies that appropriate ARA is important for larval tongue sole to maintain normal rapid growth and physiological function, but deficient or excessive dietary ARA restrains their development. These results agreed well with many previous studies in several fish species( Reference Xu, Ai and Mai 3 , Reference Bessonart, Izquierdo and Salhi 7 , Reference Luo, Tan and Li 10 , Reference Willey, Bengtson and Harel 13 ). In particular, in the present study, the larvae fed 1·42 % dietary ARA had significantly higher growth and survival rates as compared to the other groups, which indicates that the optimal requirement for dietary ARA for larval tongue sole is approximately 1·42 % (dry weight). This is comparable to studies on larval gilthead sea bream( Reference Bessonart, Izquierdo and Salhi 7 ), larval European sea bass( Reference Atalah, Hernández-Cruz and Ganuza 25 ) and larval Senegal sole( Reference Villalta, Estévez and Bransden 12 ), but some other studies have reported lower requirements for dietary ARA for several fish species during larval development, such as black sea bass( Reference Rezek, Watanabe and Harel 16 ), summer flounder( Reference Willey, Bengtson and Harel 13 ) and gilthead sea bream( Reference Fountoulaki, Alexis and Nengas 69 ). These differences were probably a result of the fish species, study methods and culture systems. In addition, it was noticed that all of the treatments in the present study obtained relatively high mortalities and low growth rates, which might be related to the characteristic lethargic browsing feeding habit of larval tongue sole( Reference Ma, Liu and Xu 50 ); the results of the survival and growth rates were comparable to those in several previous studies( Reference Chang, Liang and Wang 51 , Reference Ai, Zhao and Mai 58 ).
The relative levels of ARA and EPA have also been suggested to be important for the normal growth of larval fish; the optimal dietary EPA:ARA ratio is likely species-specific, but it should probably be about 5:1( Reference Sargent, McEvoy and Estevez 70 , Reference Bell, McEvoy and Estevez 71 ). In the present study, it is noteworthy that the dietary EPA:ARA ratio in the best growth and survival group was quite low (0·59, shown in Table 2), which is similar to other studies on ARA nutrition in larval gilthead sea bream( Reference Bessonart, Izquierdo and Salhi 7 ) and summer flounder( Reference Willey, Bengtson and Harel 13 ). However, in the present study, the EPA:ARA ratio of larval tongue sole with the best growth and survival was much higher (2·82, shown in Table 7) than in the diets. This indicates that the dietary EPA:ARA ratio might be of less importance than dietary ARA concentration for larval tongue sole, seeing as larval tongue sole were seemingly able to regulate the ratio of EPA:ARA in their bodies, even though a low dietary EPA:ARA ratio was provided.
Previous studies demonstrated that PUFA was conserved preferentially at the expense of SFA and MUFA( Reference Rainuzzo, Reitan and Jorgensen 14 , Reference Falk-Petersen, Sargent and Fox 72 ). Specific fatty acids are either selectively retained or metabolised, and preferential metabolism often occurs when a particular fatty acid is supplied at high concentrations in the diet( Reference Karalazos, Bendiksen and Dick 73 ). In the present study, SFA, n-6 PUFA and EPA in the larvae were declined as compared to those in the diets, but MUFA showed a contrary trend. This implied that the SFA, n-6 PUFA and EPA provided in the diets were possibly abundant for larval tongue sole and metabolised preferentially, and part of the SFA might be desaturated and elongated to form MUFA. In addition, higher n-3:n-6 PUFA and DHA:EPA ratios in the larvae were observed as compared to the diets, which reflects the conservation priority of n-3 to n-6 PUFA and DHA to EPA. This conformed with previous studies( Reference Watanabe 74 – Reference Sharma, Kumar and Sinha 76 ). Moreover, in the present study, increased dietary ARA concentration resulted in a concomitant increase of ARA in larvae, which is similar to reports in other fish species( Reference Bransden, Cobcroft and Battaglene 6 , Reference Villalta, Estévez and Bransden 12 ). Meanwhile, the higher ARA concentration in the diets than in the fish also indicates that except when it was integrated into the membranes, part of the exogenous ARA was probably metabolised preferentially through β-oxidation for energy production when it was supplied at high concentrations in the diet( Reference Karalazos, Bendiksen and Dick 73 ). Despite almost constant EPA in the diets, the EPA contents of the larvae in the present study generally declined in a linear manner as dietary ARA increased. This agreed well with investigations on summer flounder( Reference Willey, Bengtson and Harel 13 ), gilthead seabream( Reference Van Anholt, Koven and Lutzky 23 ), Senegal sole( Reference Villalta, Estévez and Bransden 12 ) and Japanese seabass( Reference Xu, Ai and Mai 3 ). Atalah et al. ( Reference Atalah, Hernández-Cruz and Benítez-Santana 17 ) proposed that dietary ARA was more efficiently incorporated into larval tissues than EPA, which could be related to a higher affinity of TAG and phospholipid biosynthesis enzymes stimulated by n-6 fatty acids( Reference Caballero, Gallardo and Robaina 77 ), and the increased incorporation of ARA into the larval lipids slightly reduced EPA incorporation when the latter was high in the diet.
To some extent, digestive enzyme activity could reflect the development of the larval digestive tract, and the ability of larvae to assimilate the required nutrients generally depends on the capacity of their digestive tracts to modulate digestive enzymes and metabolic processes( Reference Cahu and Zambonino Infante 78 ). Digestive enzyme activity in fish varies among species, but it can be influenced by age as well as by the quantity and composition of the diet( Reference Péres, ZamboninoInfante and Cahu 79 ). Surprisingly, however, no information is available on the effects of dietary ARA concentrations on digestive enzyme activity in larval fish. The exocrine pancreas synthesises and secretes several enzymes, including proteases, lipases and amylases, in the intestinal lumen( Reference Zambonino Infante and Cahu 80 ). In vertebrates, the protease precursor trypsinogen is synthesised and stored in the pancreas, and it is rapidly converted into active trypsin when it is released into the intestine( Reference Fange, Grove, Hoar, Randall and Brett 81 ). The acquisition of an efficient secretory function in the pancreas characterises the maturation of pancreas( Reference Tovar, Zambonino and Cahu 82 ). In the intestine, an increase in the folding of the mucosa is concomitant with a strong elevation in the activity of some digestive enzymes located in the cell membranes( Reference Ribeiro, Zambonino-Infante and Cahu 59 ). ALP and LAP, both of which are intestinal enzymes, are located in the BBM of enterocytes( Reference Cahu and Zambonino Infante 78 ). ALP is believed to be involved in the absorption of nutrients such as lipids, glucose, Ca and inorganic phosphate( Reference Tengjaroenkul, Smith and Caceci 83 ), and a strong increase in ALP activity reflects the development of the BBM of enterocytes, which occurs concurrently with a decrease in cytosolic enzymes( Reference Ribeiro, Zambonino-Infante and Cahu 59 ). LAP is often viewed as a cell maintenance enzyme that plays critical role in the turnover of peptides and the final step in protein degradation, which shows high activity throughout the larval phase( Reference Matsui, Fowler and Walling 84 ). An increase in aminopeptidase N in the intestinal BBM characterises the normal maturation of the enterocytes in fish larvae( Reference Zambonino Infante and Cahu 80 ).
Previous studies have reported that trypsin secretion was positively regulated by the dietary protein level as well as the chain length and maturity of the pancreas( Reference Zambonino Infante and Cahu 80 , Reference Cahu, Rønnestad and Grangier 85 ). Dietary protein level and chain length were therefore kept the same in the present study. The larvae fed 1·07–1·42 % dietary ARA showed higher trypsin activity in both the PS and IS than those in the other treatments, which indicates that moderate dietary ARA contributes to a better maturation and functionality of the pancreas, and insufficient or excessive ARA might delay pancreas maturation. In fish species, lipase is secreted by the hepatopancreas mainly in response to the presence of TAG in the lumen( Reference Zambonino Infante and Cahu 86 ). Cahu et al. ( Reference Cahu, Zambonino Infante and Barbosa 87 ) even found that the response of lipase was positively correlated with dietary TAG levels in sea bass larvae. In the present study, indistinctive changing trends of lipase activity in both the PS and IS were observed, which were possibly related to the almost constant lipid content and mainly methylester-form lipid in all of the diets. Because a decline in amylase activity was observed during the normal maturation process of the fish larvae, the lower amylase activity in the 1·42 % dietary ARA treatment could also be considered an indicator of the maturation of the exocrine pancreas in the present study( Reference Péres, ZamboninoInfante and Cahu 79 , Reference Ma, Cahu and Zambonino 88 ). Moreover, in the present study, elevated levels of ALP and LAP activity in the 1·42 % dietary ARA treatment were observed, which implies that moderate dietary ARA also promotes the maturation of enterocytes in the BBM in developing larval tongue sole. Overall, dietary ARA seemed to play a role in regulating the development of the digestive tract and consequently influenced the growth and survival rates of larval tongue sole. However, the mechanism by which dietary ARA affected digestive tract development is unclear, and further studies are needed to investigate this mechanism.
In mammals, ARA is metabolised into eicosanoids mainly through three different pathways, namely, COX, LOX and CYP enzymes( Reference Astudillo, Balgoma and Balboa 31 ). In the present study, the partial sequences of COX-1a, COX-1b, COX-2, 5-LOX and CYP2J6-like were obtained. COX catalyses the first committed step in the biosynthesis of PG through the conversion of ARA to PGH2 ( Reference Smith, Garavito and DeWitt 89 ). COX-1 and COX-2 are two isozymes of COX( Reference O'Neill and Ford-Hutchinson 90 ). In the present study, two COX-1 genes (COX-1a and COX-1b) and one COX-2 gene were cloned in tongue sole, and this result was similar to reports on Tetraodon nigroviridis, T. rubripes and Oryzias latipes ( Reference Ishikawa, Griffin and Banerjee 91 ). The additional copies of COX-1 and COX-2 were likely the result of a teleost-specific genome duplication event( Reference Havird, Miyamoto and Choe 92 ). 5-LOX is a member of the family of LOX( Reference Ford-Hutchinson, Gresser and Young 41 ) and is the rate-limiting enzyme in LT synthesis( Reference Mehrabian, Allayee and Wong 42 ). A partial sequence of putative 5-LOX cDNA was obtained in the present study. As mentioned earlier, it was highly homologous to 5-LOX cDNA of O. niloticus (81 %), M. zebra (80 %) and T. rubripes (79 %). CYP enzymes catalyse the epoxidation of ARA to form EET( Reference Zeldin, Foley and Ma 44 , Reference Capdevila, Falck and Harris 45 ). In vertebrates, CYP2J subfamilies are recognised as catalysts of ARA metabolism in the extrahepatic tissues of many species, such as CYP2J9 in mice( Reference Qu, Bradbury and Tsao 93 ) and CYP2J1 in zebrafish( Reference Wang, Yao and Chen 46 ). However, in the present study, only a partial sequence of a cDNA (CYP2J6-like) encoding a putative CYP2J6 protein was obtained, and the function of CYP2J6-like should be identified in future experiments.
In an effort to elucidate the mechanisms by which dietary ARA modulates growth performance of larval tongue sole, the effects of dietary ARA concentrations on ARA metabolism-related gene (COX-1a, COX-1b, COX-2, 5-LOX and CYP2J6-like) expression were investigated. In the present study, no significant differences were observed in the mRNA expression of both COX-1a and COX-1b, which indicates that both COX-1 genes were not regulated by dietary ARA, at least on the transcriptional level. This conformed to the characterisation of COX-1 as a ‘housekeeping’ gene that is constitutively expressed in almost all tissues( Reference Crofford 94 ). However, it has been reported that dietary n-6 PUFA (safflower oil) could up-regulate COX-1 expression to some extent in rat mammary glands, but dietary n-3 PUFA (menhaden oil) could not do so( Reference Badawi, El-Sohemy and Stephen 95 ). Different animal species, sampling tissue and diet formulation might account for these inconsistent results. Similar to COX-1, the expression of CYP2J6-like also did not show much difference among the dietary treatments in the present study. In in vitro studies, CYP2J has been reported to be constitutively expressed in cultured human endothelial cells( Reference Fisslthaler, Popp and Michaelis 96 , Reference Bauersachs, Christ and Ertl 97 ), and CYP2J6-like mRNA in the present study might have been expressed in the same pattern. In medical research, COX-2 has been shown to be rapidly and dramatically up-regulated in inflammatory responses( Reference Crofford 94 ), and overexpressed 5-LOX has resulted in the excessive generation of pro-inflammatory LT, which may contribute to atherosclerosis and cancer( Reference Mehrabian, Allayee and Wong 42 ). However, COX-2 and 5-LOX have also been shown to express to certain levels and contribute to maintaining fundamental physiological function under normal or basal conditions( Reference Estevez, Ishikawa and Kanazawa 28 , Reference Crofford 94 ). Obviously, the expression values of COX-2 and 5-LOX mRNA in all of the treatments in the present study were nowhere near overexpression. Meanwhile, in the present study, both the COX-2 and 5-LOX mRNA expression levels varied asymmetrically in response to dietary ARA, and the maximum expression levels of COX-2 and 5-LOX occurred in the 1·42 and 1·07 % dietary ARA treatments, respectively. Higher expression of COX-2 and 5-LOX and better growth rates, survival rates and digestive tract development were found in the same treatments. Both COX-2- and 5-LOX-dependent ARA metabolites have been reported as essential to the development and maintenance of intestinal immune homeostasis( Reference Newberry, Stenson and Lorenz 98 , Reference Cortese, Spannhake and Eisinger 99 ). This implies that both COX-2 and 5-LOX probably played a role in improving digestive tract development and consequently the growth performance of larval tongue sole by altering their mRNA expression.
To conclude, dietary ARA concentration significantly influenced survival rates, growth rates, digestive enzyme activity, fatty acid composition and some ARA metabolism-related gene expression in larval tongue sole, and a relatively higher dietary ARA concentration (1·07–1·42 %) enhanced the growth performance of larval tongue sole. The regulation of dietary ARA on growth performance in fish larvae was probably involved in altering the mRNA expression levels of COX-2 and 5-LOX. Further study is required to determine the effects of dietary ARA concentrations on ARA metabolites and the mechanism that underlies the participation of ARA metabolites in physiological activities.
Acknowledgements
We thank Y. X. Li, K. Lu, Y. L. Liu, P. Yang and J. Yan for their help in manufacturing the diets as well as S. Zhang, B. Han, X. J. Dong and J. T. Guan for their help during the sampling.
The present study was supported by the National Basic Research Program of China (973 Program) (2014CB138600), the China Agriculture Research System (CARS-50-G08) and Agricultural Scientific and Technological Achievements into Capital (2010GB23600673). The China Agriculture Research System and Agricultural Scientific and Technological Achievements into Capital had no role in the design, analysis or writing of the present article.
Y. Y. designed all of the experiments, carried out the experimental work and wrote the manuscript under the direction of the project leader Q. A. and K. M.; S. L. assisted with the experimental work and manuscript writing; Q. A. also assisted with the experimental design and manuscript revision; W. X. and Y. Z. provided all of the fatty acid composition data.
There are no conflicts of interest to report.












