The most common intervention performed by physicians is the writing of a prescription. All elements in the complex process of prescribing and administering drugs are susceptible to error (Reference Ferner and UptonFerner & Upton, 1999). Bates et al (Reference Bates, Cullen and Laird1995) reported 6.5 adverse drug events per 100 patients admitted to a Boston hospital, over a quarter of which were preventable. Drug errors are an important cause of morbidity, accounting for one-fifth of the deaths due to adverse drug events, and are therefore becoming an increasingly common subject for litigation (Reference FernerFerner, 1995).
Department of Health guidelines advise that legal responsibility for prescribing lies with the doctor who signs the prescription and the British National Formulary (BNF; British Medical Association & Royal Pharmaceutical Society of Great Britain, 1999) has explicit guidance on prescription writing.
An audit into the effects of introducing accessible hospital prescribing guidelines for opioid analgesia demonstrated an improvement in prescribing practice (Reference Humphries, Counsell and PedianiHumphries et al, 1997). Similarly Hollingsworth and Wilson (Reference Hollingsworth and Wilson1997) in a primary care study showed that good compliance with standards is achievable.
Aims
-
(a) To measure the extent to which information recorded on in-patient prescription cards conforms to South Birmingham Mental Health NHS Trust regulations (1998) for the prescribing, handling, custody and administration of drugs. The standards are based on the Medicines Act 1968, The Misuse of Drugs Act 1971, Duthie Report 1998, UKCC Advisory Paper on Administration of Medicines 1992 and also conforms to the section in the BNF on prescription writing.
-
(b) To assess the knowledge of medical staff within the older adults directorate of the trust's drug policy.
-
(c) To improve prescribing, recording and staff knowledge via a targeted series of interventions.
The study
An audit tool was designed to ascertain whether each of the standards, as outlined in the results tables, were met on each prescription card. Questions marked were areas not specified in the standard but considered to be ideal by the authors (Tables 1,2,3). Prescription cards of all the patients present on the three acute psychogeriatric wards at the Queen Elizabeth Psychiatric Hospital, Birmingham, as of midnight on 10 January 1999, were examined. Each card was examined by at least two members of the multi-disciplinary audit team. Cards had to conform throughout to meet the standard. Questionnaires were handed to medical staff to ascertain whether they had been made aware of the trust's drug policy, had read it and knew where to locate it.
Table 1. Patient identification and Mental Health Act (MHA) details
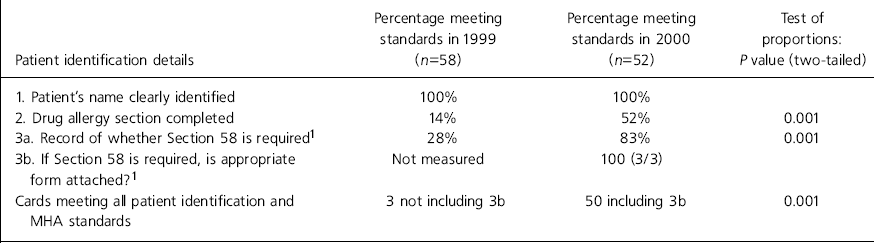
| Patient identification details | Percentage meeting standards in 1999 (n=58) | Percentage meeting standards in 2000 (n=52) | Test of proportions: P value (two-tailed) |
|---|---|---|---|
| 1. Patient's name clearly identified | 100% | 100% | |
| 2. Drug allergy section completed | 14% | 52% | 0.001 |
| 3a. Record of whether Section 58 is required1 | 28% | 83% | 0.001 |
| 3b. If Section 58 is required, is appropriate form attached?1 | Not measured | 100 (3/3) | |
| Cards meeting all patient identification and MHA standards | 3 not including 3b | 50 including 3b | 0.001 |
Table 2. Regular prescription medication details
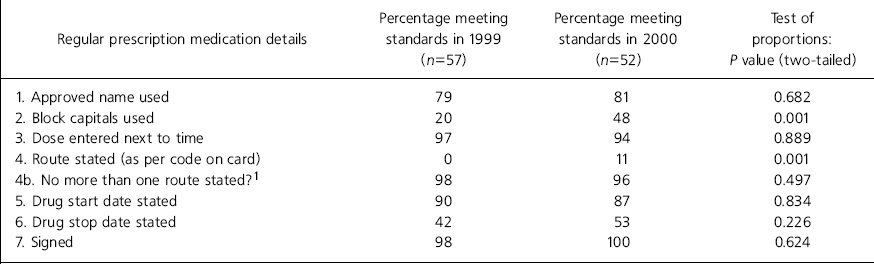
| Regular prescription medication details | Percentage meeting standards in 1999 (n=57) | Percentage meeting standards in 2000 (n=52) | Test of proportions: P value (two-tailed) |
|---|---|---|---|
| 1. Approved name used | 79 | 81 | 0.682 |
| 2. Block capitals used | 20 | 48 | 0.001 |
| 3. Dose entered next to time | 97 | 94 | 0.889 |
| 4. Route stated (as per code on card) | 0 | 11 | 0.001 |
| 4b. No more than one route stated?1 | 98 | 96 | 0.497 |
| 5. Drug start date stated | 90 | 87 | 0.834 |
| 6. Drug stop date stated | 42 | 53 | 0.226 |
| 7. Signed | 98 | 100 | 0.624 |
Table 3. Details specific to pro re nata (PRN) medication
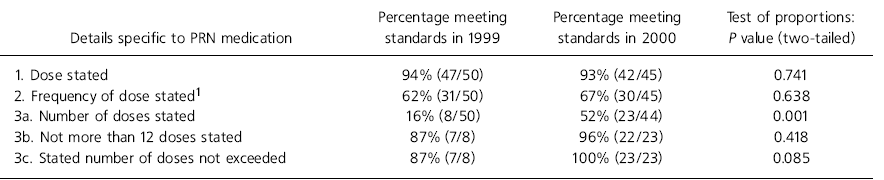
| Details specific to PRN medication | Percentage meeting standards in 1999 | Percentage meeting standards in 2000 | Test of proportions: P value (two-tailed) |
|---|---|---|---|
| 1. Dose stated | 94% (47/50) | 93% (42/45) | 0.741 |
| 2. Frequency of dose stated1 | 62% (31/50) | 67% (30/45) | 0.638 |
| 3a. Number of doses stated | 16% (8/50) | 52% (23/44) | 0.001 |
| 3b. Not more than 12 doses stated | 87% (7/8) | 96% (22/23) | 0.418 |
| 3c. Stated number of doses not exceeded | 87% (7/8) | 100% (23/23) | 0.085 |
Following the initial audit, measures were introduced to target prescription-writing skills. The results were presented to nursing, medical and management groups. A discussion took place during the in-house medical staff teaching programme related to the role of the pharmacy department in monitoring prescription and writing. Following this, a draft protocol for the role of pharmacy in ward rounds was constructed and subsequently used by the senior pharmacist. This protocol particularly includes checking that prescriptions are written according to the standards and monitoring the review of when required (pro re nata; PRN) medications.
Following these interventions the prescription cards of all patients present on the same three wards as of midnight on 13 January 2000 were evaluated by the same criteria.
Findings
In the first phase of the audit cycle 58 prescription cards were reviewed, and 52 in the second phase. No cards met all the standards in the first phase of the audit but in the second phase one card (2%) met all the standards (P=0.289). Table 1 indicates that patient identification and Mental Health Act (MHA) details were recorded correctly in 2/58 (3%) cards in phase 1 and in 26/52 (50%) cards in phase 2 (P=0.001).
In phase 1 none of the prescription cards met all the regular medication prescription details' standards but in phase 2 10% (5/52) met these standards (P=0.001). Details of individual items are shown in Table 2. In relation to the standard specific to PRN medication, in phase 1 6% of cards (3/50) met all. In phase 2 38% (17/45) met these standards (P=0.001). Details of individual items are shown in Table 3. Drug administration records were complete in 51% (29/57) of cards in phase 1 and in 61% (30/49) of cards in phase 2 (P=0.285).
In the first phase none of the three wards could locate a copy of the drug policy when asked, but all could at the re-audit. The majority of doctors knew that a drug policy was in place (8/10 at first audit, 5/6 at re-audit). There had been an improvement in the number of doctors who had read the drug policy at the second phase (2/10 at first audit, 4/6 at re-audit). In the first audit 7/10 said that they knew where to find a copy of the drug policy, whereas 3/6 did in the re-audit.
Discussion
This audit demonstrated some statistically significant improvements in prescribing practice. Pharmacists can have an important role in maintaining the quality of prescribing. Continuous evaluation and feedback have been shown to improve prescription writing (Reference Shaughnessy and D'AMICOShaughnessy & D'Amico, 1994) and generally resident doctors are amenable to receiving such information (Reference Anastasio and SigmonAnastasio & Sigmon, 1990). There was a non-significant improvement in the completion of administration records, but 39% remained incomplete in phase 2. This remains a cause for concern because potentially it could lead to drugs being given twice. It is unclear why this shortcoming persisted and more specific interventions directed at nursing staff may be indicated. Pharmacists, prescribers and nurses must institute safeguards in their practice to reduce the incidence of medication omissions.
Little emphasis is placed on prescription writing in medical training (Reference Walson, Martin and EndowWalson et al, 1981). Trainees rotate 6 monthly, allowing insufficient time to become fully familiar with drug policies, although thorough induction programmes can help to offset these difficulties (Reference Humphries, Counsell and PedianiHumphries et al, 1997). Drug policies need to be easily available and pointed out to new staff. Trainee involvement in audit is a method of raising awareness and encouraging critical evaluation of prescribing practice.
The NHS trusts should review their prescription cards to ensure that they are user friendly. Sufficient space must be provided to record relevant information, thereby facilitating compliance with standards. Approved codes for drug route, for example, need to be consistent between the standards and the key on the drug card.
There were certain limitations to this study. No attempts were made to grade the severity of the errors. Letters were sent to wards and doctors, informing them about the re-audit as a matter of courtesy. Raising awareness was a valuable part of the intervention because knowledge that practice is being observed improves performance (Reference Shaughnessy and D'AMICOShaughnessy & D'Amico, 1994). The 110 prescription cards represented the work of a small number of doctors, not all of whom participated in both phases. Despite these limitations, audit appears to have been a valuable tool for monitoring compliance to prescribing and administration standards and for encouraging continued improvement in practice.
Acknowledgements
We would like to acknowledge Staff Nurse Janet Lewis who was part of the re-audit team, Professor Femi Oyebode for his comments and the staff at South Birmingham Mental Health Trust Mental Health Services for Older Adults for their help and cooperation.






eLetters
No eLetters have been published for this article.