Adequate maternal nutrition is important for normal fetal growth and development, as the mother’s nutrient stores are the only source of nutrition for the growing fetus( Reference Marangoni, Cetin and Verduci1 ). There is an increasing interest in recent years to examine influences of maternal nutrition on cognitive development in infants, due to the growing body of literature showing a connection between improved maternal nutrition and structural changes and maturation of the infant brain( Reference Prado and Dewey2 ), which may subsequently affect early childhood cognitive function due to the strong link between anatomical changes of the brain and cognitive development( Reference Ghosh, Kakunoori and Augustinack3 ). However, there is still a limited understanding on the specific nutrients important for neurodevelopment in utero, and their subsequent longer-term effects on children’s cognitive function.
Vitamin B12 plays an important role in neural myelination, synaptogenesis and neurotransmitter synthesis( Reference Pepper and Black4 ). Myelination and synaptogenesis begin in utero and continue to influence neuronal development in offspring during the first few years of life( Reference Thompson and Nelson5 ); thus maternal vitamin B12 has the potential to affect cognitive development and function in early childhood. For example, maternal vitamin B12 deficiency may result in a disruption in myelination and synaptic connectivity in the fetal brain( Reference Pepper and Black4 ). If the development of the hippocampus, the auditory and visual cortices is impacted, memory, language and visual processing in children will consequently be affected( Reference Black6 ).
The evidence supporting the role of maternal vitamin B12 in cognitive development in children is growing. Observational studies in 1–2-year-old infants found maternal vitamin B12 deficiency to be associated with poorer mental development measured with the Bayley Scale of Infant and Toddler Development (BSID)( Reference del Rio Garcia, Torres-Sanchez and Chen7 , Reference Bhate, Joshi and Ladkat8 ), but one other observational study and a randomised controlled trial found no significant association of maternal vitamin B12 status( Reference Wu, Dyer and King9 ) or effect of maternal vitamin B12 supplementation( Reference Srinivasan, Thomas and Kapanee10 ), with/on infants’ cognition measured with BSID. Two studies in older children of 7–8 years of age found no significant associations between maternal vitamin B12 and child’s intelligence quotient( Reference Boeke, Gillman and Hughes11 , Reference Bonilla, Lawlor and Taylor12 ). In stark contrast, two studies reported higher maternal vitamin B12 concentrations or intakes to be associated with lower receptive vocabulary( Reference Villamor, Rifas-Shiman and Gillman13 ) or verbal ability( Reference Veena, Krishnaveni and Srinivasan14 ) in 3- and 10-year-old children, respectively, while another study had conflicting findings, reporting children of mothers in the lowest decile of vitamin B12 concentrations to perform poorer in a working memory task but performed better in a sustained-attention task at 9 years of age, compared with children of mothers in the highest decile of concentrations( Reference Bhate, Deshpande and Bhat15 ). Taken together, the evidence on maternal vitamin B12 and child’s cognitive outcomes is inconclusive. It was also noted that majority of studies were conducted in Western settings( Reference del Rio Garcia, Torres-Sanchez and Chen7 , Reference Wu, Dyer and King9 , Reference Boeke, Gillman and Hughes11 , Reference Bonilla, Lawlor and Taylor12 , Reference Villamor, Rifas-Shiman and Gillman13 ) or from a developing Asian country – India( Reference Bhate, Joshi and Ladkat8 , Reference Veena, Krishnaveni and Srinivasan14 , Reference Bhate, Deshpande and Bhat15 ). No studies have been conducted in a multi-ethnic (Chinese, Malay and Indian) Asian population of a developed nation which differ in socio-demographic structure, cultural environment and dietary practices.
Vitamin B12 is interconnected with folate and vitamin B6 in the one-carbon metabolism( Reference Rush, Katre and Yajnik16 ). As such, synthesis and metabolism of vitamin B12 may be influenced by the availability of these other B vitamins. There is evidence to suggest that vitamin B12 deficiency co-occurs with other B-vitamin deficiencies( Reference Simpson, Bailey and Pietrzik17 ), while several other studies reported high maternal folate coupled with low vitamin B12 to be associated with a number of infant health outcomes( Reference Swaminathan, Thomas and Kurpad18 ). Few studies to date have accounted for the influence of other B vitamins when examining maternal vitamin B12 and offspring cognition. Those that do would adjust for maternal folate in statistical model or examine interactions between maternal folate and vitamin B12 ( Reference Villamor, Rifas-Shiman and Gillman13 , Reference Veena, Krishnaveni and Srinivasan14 ), but have found to not change the associations. Interestingly, the effects of co-occurrence of maternal B-vitamin deficiencies on cognitive function in children have not been well elucidated.
In view of the aforementioned reasons, we aim to: (1) associate maternal vitamin B12 concentrations with offspring cognitive, language and motor outcomes at 24 months of age in a developed country of multi-ethnic Asians – Singapore; and (2) explore the effects of combinations of maternal vitamin B12 and folate or vitamin B6 status on child’s cognitive development.
Methods
Subjects
We used data from the GUSTO (Growing Up in Singapore Towards healthy Outcomes) study, a mother–offspring cohort study which has collected lifestyle and health information from pregnant women and their offsprings from birth onward. The GUSTO methodology has been published in detail elsewhere( Reference Soh, Tint and Gluckman19 ). In summary, pregnant women aged 18–50 years (n 1247) were recruited in their first trimester from the KK Women’s and Children’s Hospital and National University Hospital in Singapore from June 2009 to September 2010. Inclusion criteria included the following: intention to live in Singapore for the following 5 years and to deliver in one of the two study maternity units; willingness to donate birth tissues; and homogeneous ethnicity of the participants’ and spouse’s parents. The major exclusion criterion was having a pre-pregnancy health condition such as type 1 diabetes, undergoing chemotherapy, or receiving psychotropic drugs. The GUSTO cohort study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures were approved by the National Healthcare Group Domain Specific Review Board (reference D/09/021) and the SingHealth Centralised Institutional Review Board (reference 2009/280/D). Written informed consent was obtained from all participants before being enrolled into the study.
Participants for the current study were limited to the subset of mother–offspring pairs in which the mothers had plasma B-vitamin concentrations measured at 26–28 weeks’ gestation, and their offspring completed the cognitive test at 24 months of age. Due to limited manpower and available test slots, priority in scheduling to complete the cognitive test was given to infants who had participated in neurodevelopmental assessments prior to 24 months, or infants whose parents expressed interest to participate( Reference Cai, Pang and Low20 ). Those who did not participate were generally due to busy schedules, lack of interest, inability to contact the participants or participant dropping out from the GUSTO study. Further detail on the sample selection has been previously described( Reference Cai, Pang and Low20 ).
Maternal plasma B vitamins
Pregnant women underwent a venipuncture in a fasting state during the 26–28 weeks’ gestation clinic visit. The blood samples were processed within 4 hours and stored at −80°C before analysis. Plasma vitamin B12 and folate were assessed by competitive electrochemiluminescence immunoassay (ADVIA Centaur Immunoassay System; Siemens) at the NUH Referral laboratory. Between-assay CV for plasma vitamin B12 and folate were 4–9% and 6–11% respectively. Plasma vitamin B6 was analysed by using the reverse-phase HPLC method with post–column derivatisation and fluorimetric detection (MRC Human Nutrition Research, Elsie Widdowson Laboratory). Between-assay CVs was <5%.
We also measured plasma homocysteine, a functional marker of vitamin B12 status, as it has been identified to be a more sensitive indicator of vitamin B12 deficiency. Plasma homocysteine was determined using HPLC (1100 series, Agilent Technologies) and mass-spectrometry (API 3000, AB Sciex) as described by Midttun et al. (Reference Midttun, Kvalheim and Ueland21) at the Bevital AS laboratory. The between-assay CV was <2%.
Maternal dietary intake
Maternal diet during pregnancy (at 26–28 weeks’ gestation) was assessed using a 24-hour recall by trained clinical staff to obtain intakes of foods high in vitamin B12 (animal-based protein foods, e.g. poultry, meat, eggs, fish and seafood; dairy products, e.g. milk, yoghurt and cheese), and to assess overall diet quality with the Healthy Eating Index for pregnant women in Singapore (HEI-SGP)( Reference Han, Colega and Quah22 ).
Cognitive outcomes in infants
The Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III)( Reference Bayley23 ), was administered to infants at 24 (± 1) months. It is a standardised test that assesses development of children 1–42 months of age in the following domains: cognitive, receptive and expressive language, and fine and gross motor( Reference Bayley23 ). The test was performed in homes when infants were likely to be alert. Distractions were kept to a minimum (e.g. television off, a quiet space), and there were at least one parent or guardian present.
The BSID-III was administered in English, Chinese, Malay or Tamil languages depending on the child’s dominant language. As per common practice by Singapore’s clinical psychologists, the BSID-III was informally adapted into Chinese, Malay and Tamil equivalents, and scored as follows: a correct score is given for responses in a dominant language, a mix of dominant or non-dominant languages, or entirely in a non-dominant language( Reference Goh, Tham and Magiati24 ). Previous study has shown minimal influence of cultural or language bias on test performance( Reference Goh, Tham and Magiati24 ). Administration and scoring was performed by research coordinators of the same ethnicity to the child, and they were trained by the head psychologist from KKH in accordance to the manual. Training details have been described elsewhere( Reference Goh, Tham and Magiati24 ). Raw test scores were used as age-specific norms were not available for our population.
Covariates
Covariates were selected based on previous literature( Reference Boeke, Gillman and Hughes11 , Reference Bonilla, Lawlor and Taylor12 , Reference Villamor, Rifas-Shiman and Gillman13 , Reference Veena, Krishnaveni and Srinivasan14 ). Information on maternal age and self-reported ethnicity and highest education attained was collected during recruitment visit (<14 weeks’ gestation). At the 26–28 weeks’ gestation clinic visit, information on antenatal mental well-being assessed with the Edinburgh Postnatal Depression Scale( Reference Cox, Holden and Sagovsky25 ) (Cronbach’s alpha for internal reliability = 0·82) and the State-Trait Anxiety Inventory ( Reference Spielberger26 ) (Cronbach’s alpha for internal reliability = 0·91); an oral-glucose-tolerance test was also administered, and the diagnosis of gestational diabetes mellitus was based on the 1999 WHO criteria( Reference Alberti and Zimmet27 ). Maternal pre-pregnancy BMI was based on self-reported pre-pregnancy weight and height measured with a stadiometer (SECA model 213) at the 26–28 weeks’ gestation clinic visit, calculated as weight divided by height squared (kg/m2). Maternal parity and infant sex were retrieved from hospital delivery records.
Statistical analysis
The BSID-III raw scores were converted to standard deviation scores to facilitate comparison across the domain subscales. Maternal vitamin B12 statuses during pregnancy were categorised as follows: deficient (<148 pmol/l), insufficient (148 to <221 pmol/l) and sufficient (≥221 pmol/l), based on commonly used cut-offs in literature( Reference Simpson, Bailey and Pietrzik17 , 28 ). For maternal homocysteine, the top 75th percentile of the study sample was used to define high concentrations of homocysteine (≥5·5 µmol/l) as none of the mothers had plasma homocysteine concentrations above the cut-off for elevated homocysteine (>10 µmol/l)( Reference Veena, Krishnaveni and Srinivasan14 , Reference Bhate, Deshpande and Bhat15 , Reference Kvestad, Hysing and Shrestha29 ).
Maternal and infant characteristics according to maternal vitamin B12 status were compared using the χ2 test for categorical variables, and using one-way ANOVA or Kruskal–Wallis tests for continuous variables with normal or skewed distribution, respectively. Associations between maternal vitamin B12 and homocysteine status and scores of each BSID-III subscale in the infants were examined using linear regressions. Several statistical models were employed: Model 1 – basic model with adjustment for infant’s exact age at cognitive testing; Model 2 – additional adjustment for maternal age, ethnicity, education, pre-pregnancy BMI, parity, gestational diabetes mellitus status and antenatal depression and anxiety levels; and Model 3 – further adjustment for maternal plasma folate and vitamin B6 concentrations, and additionally for maternal plasma vitamin B12 concentrations for homocysteine analysis. Potential effect modifications by infant sex on the associations between maternal vitamin B12 and infant’s BSID-III outcomes were also explored.
As vitamin B12 deficiency tends to co-occur with folate and vitamin B6 deficiencies, we further explored combinations of maternal vitamin B12 and vitamin B6 or folate status in relation to infant BSID-III subscale scores. For a simpler analysis, we re-classify mother–infant pairs into two groups of maternal vitamin B12 status: insufficient B12 (<221 pmol/l) and sufficient B12 (≥221 pmol/l). Mother–infant pairs were also grouped according to maternal vitamin B6 and folate status: insufficient B6 (<20 nmol/l) and sufficient B6 (>20 nmol/l)( Reference Coburn, Lewis and Fink30 ); insufficient folate (<13·6 nmol/l) and sufficient folate (≥13·6 nmol/l)( Reference Oner, Vatansever and Karasalihoglu31 ). For combinations of maternal vitamin B12 and B6 statuses, the reference group is mothers who were sufficient in both B12 and B6, while the comparison groups are mothers who were (i) insufficient in both B12 and B6 and (ii) sufficient in B6 but insufficient in B12. For combinations of maternal vitamin B12 and folate statuses, the reference group is mothers who were sufficient in both vitamin B12 and folate, while the comparison groups are mothers who were (i) insufficient in both B12 and folate and (ii) sufficient in folate but insufficient in B12. Groups with very small sample sizes, which we hypothesised to be the following: (i) sufficient in vitamin B12 but insufficient in folate and (ii) sufficient in vitamin B12 but insufficient in vitamin B6, will be excluded from the analysis of B12–B6 and B12–folate combinations. The statistical models for this analysis were adjusted for covariates as per Model 3 discussed earlier.
Missing data for covariates were imputed using multiple imputation techniques with chained equations (twenty times). All analyses were performed using Stata version 14 (StataCorp LP). The significance level was set at P<0·05.
Results
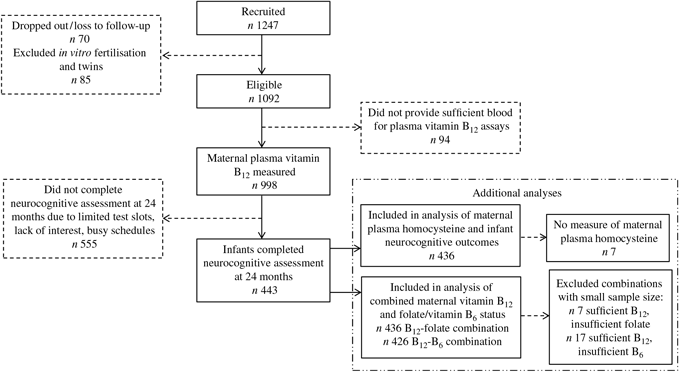
Of the 1247 pregnant women initially recruited, 70 dropped out during pregnancy due to personal reasons or family disapproval, or loss to follow-up; eighty-five conceived through in-vitro fertilisation or gave birth to twins and were excluded. A total of 1092 of remaining women conceived naturally with singleton foetuses, and 998 provided sufficient blood for assays of plasma vitamin B12, folate and vitamin B6 concentrations. A subset of their offspring (n 443) completed the BSID-III at 24 months of age (Fig. 1). This subset of mother–offspring pairs was included in the maternal vitamin B12 and offspring neurocognitive outcomes. The 555 mother–offspring pairs who did not participate in the BSID-III were comparable in characteristics to those who participated (online Supplementary Table S1). The analysis for maternal homocysteine was performed in 436 mother–offspring pairs as seven mothers had no measurement for homocysteine. The analysis examining combinations of maternal vitamin B12 and folate or vitamin B6 status was performed in 436 and 426 mother–offspring pairs respectively; seven mothers who were vitamin B12 sufficient but folate insufficient and seventeen mothers who were vitamin B12 sufficient but vitamin B6 insufficient were excluded.

Fig. 1. Participant flow diagram for analysis of associations between maternal plasma vitamin B12 concentrations and infant cognitive development in the Growing Up in Singapore Towards healthy Outcomes study.
Characteristics of mother–offspring pairs
Maternal and infant characteristics according to maternal vitamin B12 status are presented in Table 1. A total of 15·6 % of mothers were vitamin B12 deficient and 41·8 % of mothers were vitamin B12 insufficient. Mothers who were vitamin B12 deficient were more likely to belong to the Indian ethnic group, tended to have higher concentrations of homocysteine and more likely to have lower concentrations of vitamin B6 and folate as well as a greater proportion of them having insufficient vitamin B6 and folate. These mothers were also observed to have higher pre-pregnancy BMI and tended to be primi- or multi-parous. In addition, mothers with vitamin B12 deficiency or insufficiency tended to have lower intakes of meat, eggs or animal-based products and dairy products, although the groups of mothers did not differ in their overall diet quality.
There were missing observations for the following variables: n 2 maternal education, n 8 antenatal depression, n 7 antenatal anxiety, n 36 maternal pre-pregnancy BMI, n 14 maternal gestational diabetes mellitus, n 3 animal-based protein foods and n 3 dairy products.
Table 1. Maternal and infant characteristics according to maternal vitamin B12 status in 443 mother–offspring pairs of the Growing Up in Singapore towards healthy Outcomes (GUSTO) cohort (Numbers of participants and percentages; mean values and standard deviations; medians and interquartile ranges (IQR))
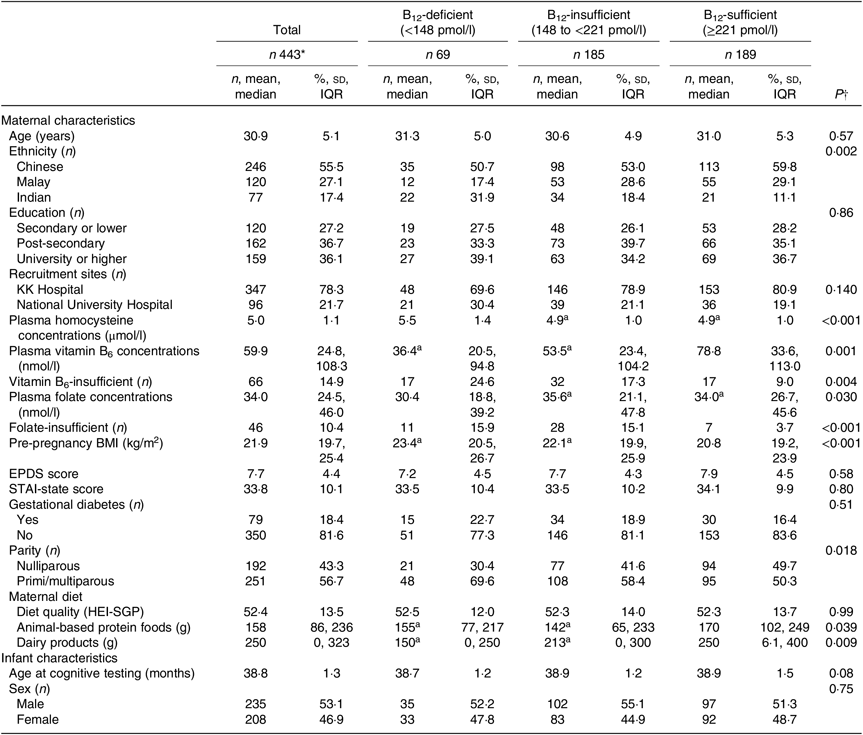
EPDS, Edinburgh Postnatal Depression Scale; STAI, State-Trait Anxiety Inventory; HEI-SGP, Healthy Eating Index for Singapore Pregnant women.
a Groups with the same superscript letter in a row indicate no significant difference in P values by one-factor ANOVA or Kruskal–Wallis test with Bonferroni post hoc analysis.
* Missing data: n 2 maternal education, n 7 maternal plasma homocysteine, n 8 antenatal depression, n 7 antenatal anxiety, n 36 maternal pre-pregnancy BMI, n 14 maternal gestational diabetes mellitus, n 3 animal-based protein food intake, n 3 dairy product intake.
† P values were obtained from the χ2 test, one-factor ANOVA or Kruskal–Wallis test with Bonferroni post hoc analysis.
Maternal vitamin B12, homocysteine and cognitive outcomes in infants
Compared with infants of mothers with sufficient vitamin B12, infants of mothers with vitamin B12 deficiency had 0·42 (95 % CI −0·70, −0·14) sd lower cognitive scores, upon adjusting for key confounders (Table 2). This association was not affected by additional adjustment for maternal plasma folate and vitamin B6 concentrations. Findings were consistent when maternal vitamin B12 concentrations were treated as a continuous variable, whereby higher maternal vitamin B12 concentrations were associated with higher cognitive scores in infants (online Supplementary Table S2).
Table 2. Associations of maternal plasma vitamin B12 status* with infant cognitive development (Bayley Scale of Infant and Toddler Development–III) at 24 months of age in the Growing Up in Singapore Towards healthy Outcomes study (n 443) (β-Coefficients and 95 % confidence intervals)
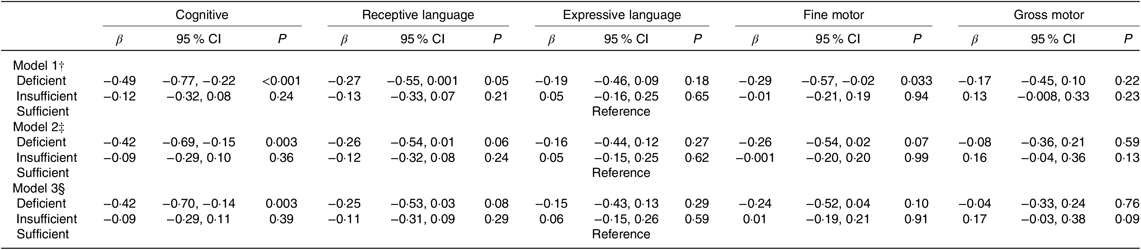
* Vitamin B12 status: n 89 deficient (<148 pmol/l); n 185 insufficient (148 to <221 pmol/l); n 189 sufficient (≥221 pmol/l).
† Model 1 – adjusted for infant’s age at cognitive testing.
‡ Model 2 – adjusted as for Model 1 and maternal age, ethnicity, education, pre-pregnancy BMI, parity, gestational diabetes status, antenatal depression and anxiety levels.
§ Model 3 – adjusted as for Model 2 and maternal plasma folate and vitamin B6 concentrations.
No significant associations were observed for maternal vitamin B12 status or concentrations with other BSID-III subscales in infants. There were no interactions between maternal vitamin B12 and infant sex in relation to each BSID-III subscales (data not shown).
Infants of mothers with high homocysteine concentrations appeared to score lower in most of the BSID-III subscales (four of five subscales) compared with infants of mothers with normal concentrations; but none of these associations reached statistical significance (Table 3).
Table 3. Associations of maternal plasma homocysteine status* with infant cognitive development (Bayley Scale of Infant and Toddler Development–III) at 24 months of age in the Growing Up in Singapore Towards healthy Outcomes study (n 436) (β-Coefficients and 95 % confidence intervals)
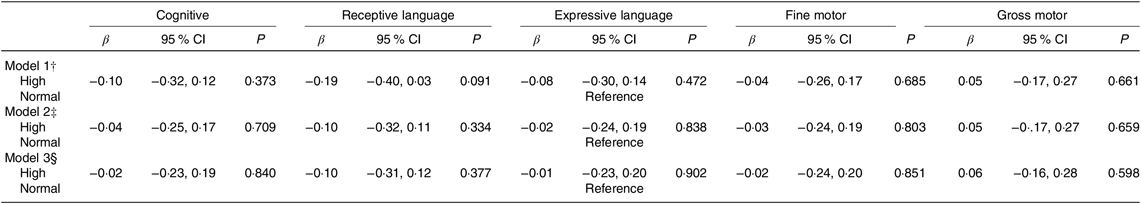
* Homocysteine status: n 117 high (≥75th percentile – ≥5·5 µmol/l;); n 326 normal (<75th percentile – <5·5 µmol/l).
† Model 1 – adjusted for infant’s age at cognitive testing.
‡ Model 2 – adjusted as for Model 1 and maternal age, ethnicity, education, pre-pregnancy BMI, parity, gestational diabetes status, antenatal depression and anxiety levels.
§ Model 3 – adjusted as for Model 2 and maternal plasma vitamin B12, folate and vitamin B6 concentrations.
Combined maternal vitamin B12 and folate or vitamin B6 status with cognitive outcomes in infants
When compared with infants of mothers who were sufficient in both vitamins B12 and B6 (reference group), infants of mothers who were insufficient in both vitamins B12 and B6 had 0·37 (95 % CI −0·69, −0·06) sd lower cognitive score, while nostatistical significant association was observed for infants of mothers with insufficient B12 but sufficient B6 (Table 4).
Table 4. Associations of combined maternal plasma vitamins B12 and vitamin B6 or folate status* with infant cognitive development (Bayley Scale of Infant and Toddler Development–III) at 24 months of age in the Growing Up in Singapore Towards healthy Outcomes study (n 443)† (β-Coefficients and 95 % confidence intervals)
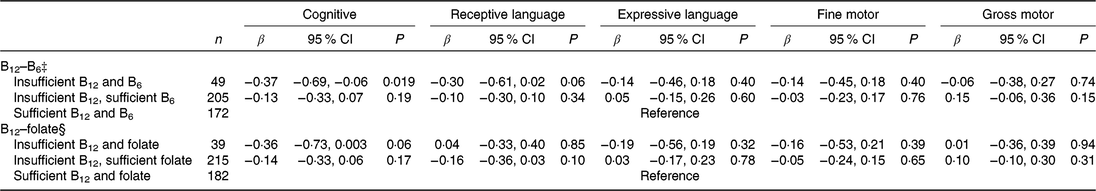
* Vitamin B12 status: insufficient (<221 pmol/l including deficient), sufficient (≥221 pmol/l); vitamin B6 status: insufficient (<20 nmol/l), sufficient (>20 nmol/l); folate status: insufficient (<13·6 nmol/l), sufficient (≥13·6 nmol/l).
† Models adjusted for infant’s age at testing; maternal age, ethnicity, education, pre-pregnancy BMI, parity, gestational diabetes status, antenatal depression and anxiety levels, and maternal plasma ‡folate or §vitamin B6 concentrations.
No significant associations were observed for combinations of maternal vitamin B12 and folate status with all BSID-III subscales in infants.
Independent of vitamin B12, however, there were no significant associations between maternal folate and vitamin B6 concentrations or status with each BSID-III subscales in infants (online Supplementary Table S3).
Discussion
Our study found infants of mothers deficient in vitamin B12 deficiency to perform less well in the cognitive domain compared with infants of mothers who were sufficient in vitamin B12. In addition, infants performed less well in the cognitive domain if their mothers had co-occurrence of vitamins B12 and B6 insufficiencies/deficiencies during pregnancy, but not if the mothers were sufficient in vitamin B6 although also insufficient/deficient in vitamin B12.
Our finding regarding the role of maternal vitamin B12 on infant’s BSID-III cognitive domain is in line with two previous birth cohort studies examining maternal vitamin B12 and cognitive development in 1–2-year-old infants measured with BSID-II or -III( Reference del Rio Garcia, Torres-Sanchez and Chen7 , Reference Bhate, Joshi and Ladkat8 ). Another cohort study in Canada, however, showed no significant associations between maternal vitamin B12 concentrations and BSID-III outcomes in their offspring at 18 months( Reference Wu, Dyer and King9 ), which may be due to a small sample size of 154 mother–infant pairs or insufficient variation in maternal vitamin B12 status given the low prevalence of deficient/insufficient vitamin B12 in their participants. One randomised controlled trial did not find significant effects of maternal B12 supplementation during pregnancy on cognitive development (also measured with BSID-III) in infants at 9 months( Reference Srinivasan, Thomas and Kapanee10 ). The lack of effect could be due to the young age at cognitive assessment which may have affected the reliability of the results.
The effect estimate of maternal vitamin B12 and infant cognitive score association in our study appears to be fairly similar to studies reporting significant associations. Previous studies found children born to vitamin B12-deficient mothers to score 1·6–3 points lower in BSID-II mental development index compared with children born to vitamin B12-sufficient mothers( Reference del Rio Garcia, Torres-Sanchez and Chen7 , Reference Bhate, Joshi and Ladkat8 ). Our study found infants of vitamin B12-deficient mothers to score two points (0·42 sd) lower in BSID-III cognitive subscale compared with infants of vitamin B12-sufficient mothers, although the differences in BSID editions, vitamin B12 measurement methods and statistical methods meant that results may not be directly comparable. The clinical significance of this effect estimate is unclear, but it is important to note that the effect size is similar to that of the association between maternal education and infant cognitive scores in our study (0·41 SD lower comparing infants of mothers with the lowest v. the highest education level), which has been identified to be a strong predictor of child’s cognition in the literature( Reference Harding, Morris and Hughes32 ).
Similar to two other studies reporting a lack of associations between maternal vitamin B12 concentration or intakes and offspring psychomotor development( Reference del Rio Garcia, Torres-Sanchez and Chen7 , Reference Bhate, Joshi and Ladkat8 ), we too did not observe any association between maternal vitamin B12 concentrations and the gross motor subscale in our infants. Studies examining vitamin B12 concentrations or intakes in children with motor development also reported similar findings( Reference Kvestad, Hysing and Shrestha29 , Reference Louwman, van Dusseldorp and van de Vijver33 , Reference Strand, Taneja and Ueland34 ). These studies, on the other hand, found significant associations with mental development and several cognitive aspects, which is consistent with our findings. Interestingly, one randomised controlled trial found vitamin B12 supplementation in infants to improve gross motor development, although the effect was attenuated after accounting for baseline differences of important confounders (e.g. sex, age, family income and physical growth)( Reference Kvestad, Taneja and Kumar35 ).
We did not find maternal vitamin B12 to be associated with offspring language development. The literature relating vitamin B12 to language development in children is inconsistent. Two studies reported inverse associations between maternal vitamin B12 and offspring receptive language( Reference Villamor, Rifas-Shiman and Gillman13 ) and verbal fluency( Reference Veena, Krishnaveni and Srinivasan14 ), while another study found no significant association between maternal vitamin B12 and offspring verbal intelligence( Reference Boeke, Gillman and Hughes11 ). Likewise, vitamin B12 supplementation in infants appears to have no effect on communication ability( Reference Kvestad, Taneja and Kumar35 ). Direct comparison of these study findings is not possible, as there is no current consensus in the instruments used to assess language development( Reference Venkatramanan, Armata and Strupp36 ).
The association between maternal B12 deficiency and lower cognitive scores appears to be more evident among mother–offspring pairs where the mothers were also vitamin B6 insufficient during pregnancy. Vitamins B12 and B6 are the sources of coenzymes which participate in one-carbon metabolism shown to play a role in neurodevelopment( Reference Dominguez-Salas, Cox and Prentice37 ); the lack of both nutrients may thus have an additive negative effect on cognitive function. Being insufficient in vitamin B6 may also contribute to malabsorption of vitamin B12 ( Reference Simpson, Bailey and Pietrzik17 ), and further contribute to impairing neurocognitive development. This is supported by our observation that mothers who were vitamin B12 deficient were also more likely to have the lowest concentrations of vitamin B6, and a greater proportion of them to have insufficient vitamin B6, indicating that these two B vitamins mutually influence the synthesis of each other. Note, however, that the group who were insufficient in both vitamins B12 and B6 were much smaller in comparison with the other two groups (sufficient in vitamins B12 and B6, and insufficient vitamin B12 but sufficient vitamin B6); the effect estimate may be biased by underpowered analysis.
Vitamin B12 is an essential nutrient not synthesised by the human body and can be obtained only through the consumption of meat and animal products or foods fortified with vitamin B12(28). This may explain our observation of mothers deficient or insufficient in vitamin B12 having significantly lower intake of animal-based protein foods and dairy products but did not differ in diet quality, as vitamin B12 concentrations are more reflective of meat and animal product intakes rather than an overall healthier diet. Concordantly, we found mothers with deficiency or insufficiency vitamin B12 tended to belong to the Indian ethnic group, and a higher proportion of them in our cohort were adopting a vegetarian diet during pregnancy (7·9% v. 1·4% Chinese and 2% Malay).
The interpretation of maternal vitamin B12 status during pregnancy is complicated by haemodilution and complex physiological changes and may not be a true reflection of inadequate dietary intake. As such, we also measured maternal plasma homocysteine, a functional biomarker of vitamin B12 status. We found vitamin B12-deficient mothers to have significantly higher plasma homocysteine concentrations, suggestive of a vitamin B12 deficiency, although the concentrations in our sample did not reach the level necessary for hyperhomocysteinemia (>10). Given that plasma homocysteine reduced by 36 % of non-pregnant values during mid-pregnancy(Reference Varela-Moreiras, Murphy and Scott38), and that homocysteine is affected by the availability of other B vitamins (e.g. folate, vitamins B6 and B2), thus may not be a specific biomarker of vitamin B12(Reference Allen, Miller and de Groot39), helps explain the disproportionate prevalences of vitamin B12 deficiency to hyperhomocysteinemia in our sample. This observation is also supported by two other studies reporting a much higher prevalence of vitamin B12 deficiency compared with the prevalence of hyperhomocysteinemia (Veena et al. (Reference Veena, Krishnaveni and Srinivasan14): 42·5% v 3·4%; Bhate et al.(Reference Bhate, Deshpande and Bhat15): 65% v. 35%). The lack of association between maternal plasma homocysteine and offspring BSID-III outcomes in our study may be explained by the absence of neurotoxic effect arising from hyperhomocysteinemia.
The present study has several strengths. First, the use of plasma B-vitamin concentrations are independent of self-reported bias and would be fairly more accurate than conventional methods of dietary assessments such as food frequency questionnaires and 24-hour recalls, which have the potential for over- or underestimation( Reference Willett40 ). We also considered the contribution of the other B vitamins involved in the one-carbon cycle to determine their level of influence on the development of cognition. Our results are robust, as they remained significant even after adjusting for several key confounders such as socio-economic status (using maternal education as proxy) and maternal mental health.
Some limitations of our study include the fact that the study is an observational study, thus no causative relationships can be drawn from the results. The analysis was performed on a subset of infants who have completed BSID-III and may lead to selection bias; comparison of participant characteristics showed that non-participants were similar in profile for a number of key determinants. Vitamin B12 concentrations were not measured in children; hence a better (or poorer) performance in neurocognitive assessments may be a reflection of better (or poorer) nutritional status in children rather than of their mothers, although there is evidence to suggest that dietary patterns of the offspring are very similar to those of their mothers( Reference Fisk, Crozier and Inskip41 , Reference Davison, Saeedi and Black42 ). A number of important contributors to early cognitive development such as maternal intelligence and home stimulation were not measured in the cohort, but our statistical models adjusted for maternal education which is often used as a proxy. Our study could benefit from having measured methymalonic acid which is a more specific functional biomarker of vitamin B12 compared with homocysteine, to provide a more comprehensive aspect of whether vitamin B12 deficiency is truly present in our population of pregnant women. Finally, study findings may be biased by how well infant’s cognitive performance is captured, but much efforts have been put in place to ensure information collected is reliable in terms of training of research coordinators, requesting for minimal distractions during administration and ensuring that infant’s performance in BSID-III is minimally influenced by cultural and language bias( Reference Goh, Tham and Magiati24 ).
In conclusion, maternal vitamin B12 deficiency was associated with poorer cognitive function in 2-year-old infants. Further studies on circulating and functional biomarkers of vitamin B12 to comprehensively assess vitamin B12 status and inclusion of multiple measures of cognitive outcomes at later time points are needed to clearly elucidate the associations between maternal vitamin B12 and cognition in children. It is also essential that the associations observed are tested in well-designed randomised controlled trials before recommending vitamin B12 during pregnancy for improved offspring cognitive development. Nevertheless, there is still a need to advise pregnant women on optimal diets to ensure adequate vitamin B12 especially those with low consumption of animal-based protein foods and dairy products, in view of the high prevalence of B12 deficiency and insufficiency (57·5 %) in our cohort.
Acknowledgements
We will like to acknowledge the contribution of the GUSTO study group: Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Christiani Jeyakumar Henry, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, Fabian Yap, George Seow Heong Yeo, Helen Chen, Hugo P. S. van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joshua J. Gooley, Keith M. Godfrey, Kenneth Kwek, Kok Hian Tan, Krishnamoorthy Niduvaje, Leher Singh, Lin Lin Su, Lourdes Mary Daniel, Lynette Pei-Chi Shek, Marielle V. Fortier, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Michael Meaney, Mya Thway Tint, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Peter D. Gluckman, Pratibha Agarwal, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, Shang Chee Chong, Shirong Cai, Shu-E Soh, Sok Bee Lim, Chin-Ying Stephen Hsu, Victor Samuel Rajadurai, Walter Stunkel, Wee Meng Han, Wei Wei Pang, Yap-Seng Chong, Yin Bun Cheung, Yiong Huak Chan and Yung Seng Lee.
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore – NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), and Nestec. K. M. G. is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and by the European Union’s Seventh Framework Programme (FP7/2007–2013), project EarlyNutrition under grant agreement n°289346. The funding bodies had no influence on the study design, data collection, analysis, interpretation and content of the manuscript.
J. S. L. and M. N. M. A. contributed to the design of the study, analysed and interpreted data, and wrote the manuscript. M. F. F. C. and A. R.-G. designed the study, reviewed and edited the manuscript. J. S. L. and M. F. F. C. had primary responsibility for final content. S. C., M. J. M. and B. F. B. P. were involved in the design of the protocol used in the cognitive assessments. P. L. Q. was involved in coordinating blood samples and nutrients data. P. D. G., L. P. S., F. Y., K. H. T., Y. S. C. and K. M. G. led the GUSTO study. All authors critically reviewed the manuscript for scientific content, read and approved the final manuscript.
F. Y., P. D. G., K. M. G. and Y. S. C. have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. P. D. G., K. M. G. and Y. S. C. are part of an academic consortium that has received research funding from Abbott Nutrition, Nestlé and Danone. J. S. L., M. N. M. A., S. C., P. L. Q., L. P. S., K. H. T., M. J. M., B. F. P. B., A. R., M. F. F. C.: ‘None’.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519000746