Introduction
Schizophrenia is a severe and chronic disease, with varying presentation, course, and response to treatment among patients. Switching antipsychotic medications is common in the management of schizophrenia, for reasons including unsatisfactory response, relapse (despite compliance), or intolerability due to adverse events.Reference Edlinger, Baumgartner and Eltanaihi-Furtmüller1–Reference Newcomer, Weiden and Buchanan4 Many different switching strategies can be conceived, according to the speed of titration and the degree of overlap between agents.Reference Correll5 Switching strategies are of varying complexity and have differing risks of drug interactions, subtherapeutic dosing, and withdrawal or rebound symptoms.Reference Edlinger, Baumgartner and Eltanaihi-Furtmüller1, Reference Buckley and Correll2, Reference Correll5, Reference Correll6 Cross-titration, involving the gradual dose escalation of a new antipsychotic with overlapping gradual discontinuation of the previous antipsychotic,Reference Correll5 is the preferred switching method for oral atypical antipsychotics according to a panel of experts in the treatment of schizophrenia.Reference Kane, Leucht, Carpenter and Docherty7 In particular, “plateau” cross-titration, involving the gradual dose escalation of new antipsychotic with delayed gradual discontinuation of the previous antipsychotic (only after a therapeutic dose of the new antipsychotic has been reached and 4–5 half-lives of the previous antipsychotic have passed), has been proposed as the best strategy to avoid rebound phenomena,Reference Correll5, Reference Correll8 although this is not supported by current evidence from randomized controlled trials that may not reflect patients and treatment strategies in clinical care.Reference Takeuchi, Thiyanavadivel, Agid and Remington9 For example, plateau cross-titration may minimize the rebound psychosis, mania, or agitation that can occur when switching from a full dopamine D2 antagonist to a partial D2 agonist, and the rebound anxiety, insomnia, agitation, and restlessness that can occur when switching from an antipsychotic with more potent antihistaminergic or anticholinergic activity to one with less antihistaminergic or anticholinergic activity.Reference Correll6, Reference Correll8 Taking into account these recommendations, individualized switching strategies should be devised based on the properties of the antipsychotics involved (e.g., efficacy, tolerability, dosing schedule, pharmacodynamics, and pharmacokinetics), as well as patient factors (e.g., past response and severity of illness).Reference Buckley and Correll2, Reference Correll8
Brexpiprazole is a serotonin–dopamine activity modulator that acts as a partial agonist at serotonin 5-HT1A and dopamine D2 receptors and as an antagonist at serotonin 5-HT2A and noradrenaline α1B/2C receptors, all with subnanomolar potency.Reference Maeda, Sugino and Akazawa10 The efficacy and safety of brexpiprazole have been demonstrated in a phase 3 schizophrenia program comprising two acute studiesReference Correll, Skuban and Ouyang11, Reference Kane, Skuban and Ouyang12 and a maintenance study.Reference Fleischhacker, Hobart and Ouyang13 Brexpiprazole is approved in the United States, Canada, Australia, and Japan for the treatment of schizophrenia, and in the United States as an adjunctive therapy to antidepressants for the treatment of major depressive disorder.
Due to the design of the acute studies for regulatory approval, in which patients were washed out from prior antipsychotics before brexpiprazole was initiated, these studies generated no data on switching strategies or outcomes.Reference Correll, Skuban and Ouyang11, Reference Kane, Skuban and Ouyang12 In contrast, the brexpiprazole maintenance study included a 1–4 week conversion phase in which patients on previous antipsychotic(s) were cross-titrated to brexpiprazole monotherapy.Reference Fleischhacker, Hobart and Ouyang13 The aim of the present analysis was to use data from the maintenance study to compare the tolerability and efficacy of different clinicians’ choice of cross-titration schedules to aid clinicians in choosing a switching schedule to brexpiprazole monotherapy that best meets their patients’ needs.
Methods
Study design and patients
This was a post hoc analysis of data from a multicenter, randomized, double-blind, placebo-controlled phase 3 maintenance withdrawal study (Equator; NCT01668797).Reference Fleischhacker, Hobart and Ouyang13 For a full description of the study design and selection criteria, please refer to Fleischhacker and colleagues’ 2017 publication.Reference Fleischhacker, Hobart and Ouyang13 The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice Guideline and local regulatory requirements. The protocol was approved by an institutional review board or independent ethics committee for each investigational site, and all patients provided written informed consent to participate.
The study included male and female patients aged 18−65 years with a diagnosis of schizophrenia for at least 3 years as defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR)14 and confirmed by the Mini International Neuropsychiatric Interview (MINI) for Schizophrenia and Psychotic Disorders Studies.Reference Sheehan, Janavs and Harnett-Sheehan15 Patients had to have been experiencing an acute exacerbation of psychotic symptoms at screening, as demonstrated by a Positive and Negative Syndrome Scale (PANSS)Reference Kay, Fiszbein and Opler16 total score of >80. Patients had to have shown a response to antipsychotic treatment (other than clozapine) in the previous year, had to be currently being treated with oral or depot antipsychotics (other than clozapine) or to have a recent lapse in antipsychotic treatment, and had to have a history of relapse and/or symptom exacerbation in the absence of antipsychotic treatment. Exclusion criteria included a DSM-IV-TR Axis I diagnosis other than schizophrenia, acute depressive symptoms in the previous 30 days requiring antidepressant therapy, antipsychotic-resistant or refractory schizophrenia, a significant risk of violent behavior or suicide, meeting DSM-IV-TR criteria for substance abuse or dependence in the previous 180 days, or requiring prohibited concomitant therapy during the trial.
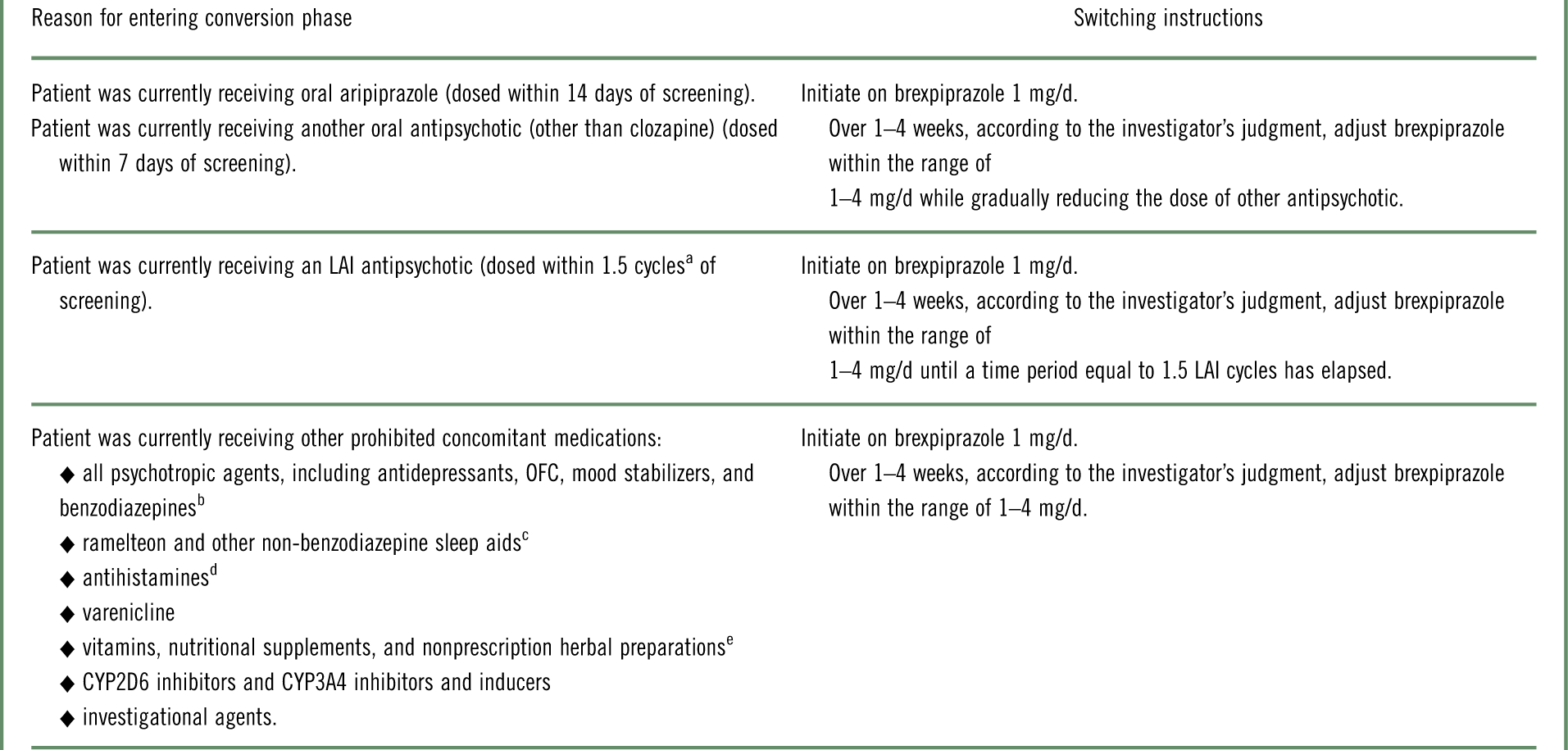
The study comprised a screening phase, an open-label phase for conversion from other antipsychotics to monotherapy with oral brexpiprazole (and washout of prohibited concomitant medications), a single-blind treatment phase to stabilize patients on oral brexpiprazole, a double-blind maintenance treatment phase (in which patients were randomized to oral brexpiprazole or placebo), and a safety follow-up phase. Following screening, eligible patients entered the conversion phase if they were currently receiving antipsychotics or other prohibited concomitant medications (Table 1); otherwise, they entered the stabilization phase directly. During the conversion phase, patients were converted to once-daily brexpiprazole over 1–4 weeks, as outlined in Table 1. Simultaneously, previous antipsychotic treatment and any prohibited medications were discontinued. Patients completing the conversion phase, and patients not requiring conversion, entered a 12–36 week stabilization phase. In this phase, patients were titrated to a dose of brexpiprazole (1–4 mg/d) that would maintain stability of psychotic symptoms while minimizing tolerability issues.
Table 1 Strategy of switching to brexpiprazole monotherapy during the conversion phase, according to antipsychotic or other prohibited concomitant medication at screening
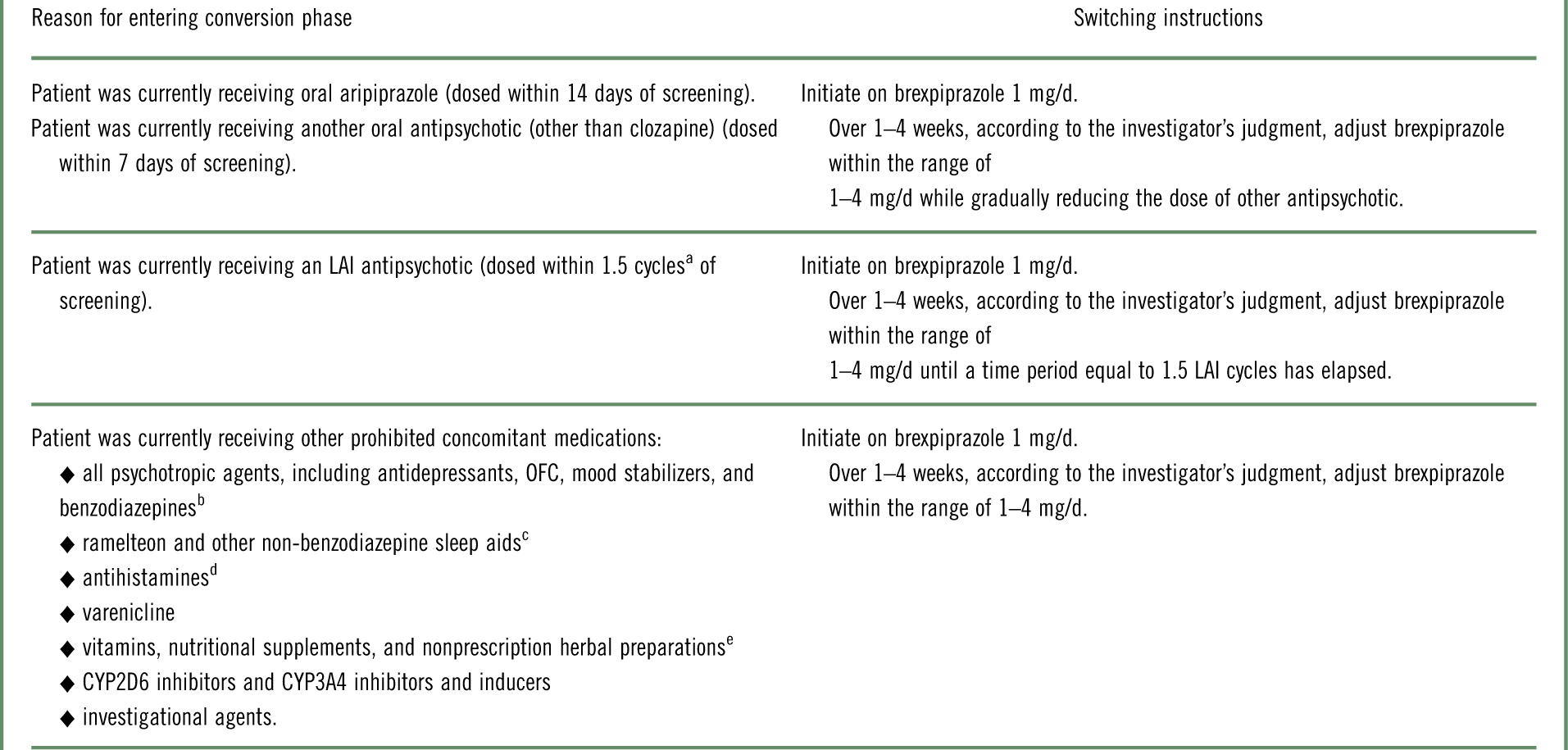
LAI=long-acting injectable; OFC=olanzapine–fluoxetine combination.
a Cycle length was based on the prescribing label. If the required washout was >28 days, the patient was kept in the screening phase for up to 14 days until ≤28 days were remaining.
b Except lorazepam or oxazepam (clonazepam or diazepam, if not available) used as a rescue medication.
c Except zolpidem, zaleplon, zopiclone, and eszopiclone for the treatment of insomnia.
d Except loratadine and cetirizine.
e Unless approved in advance by the medical monitor.
This post hoc analysis focused on a time frame of 8 weeks after the first dose of brexpiprazole, covering the conversion phase and the start of the stabilization phase. A subsample comprising all patients in whom at least 8 weeks of brexpiprazole treatment was achieved was chosen to explore: (1) which of the clinicians’ chosen switching strategies were the most represented among the successful switches from prior antipsychotic treatment to brexpiprazole; and (2) whether any baseline patient or treatment differences existed among the different naturalistically employed, successful switching strategies.
Assessments
In this post hoc analysis, tolerability was assessed by the time to, and reasons for, discontinuation, and the incidence of treatment-emergent adverse events (TEAEs). Efficacy was assessed using the PANSS and the Clinical Global Impressions–Severity of illness (CGI-S) scale. The PANSS is a clinician-rated scale that measures positive, negative, and general psychopathology symptoms, scored from 30 to 210, where a higher score indicates greater psychopathology.Reference Kay, Fiszbein and Opler16 The CGI-S is a clinician-rated scale that measures global illness severity, scored from 1 (not at all ill) to 7 (among the most extremely ill).Reference Guy17 The PANSS and CGI-S were administered at the baseline visit and last visit of the conversion phase and at every 2 weeks during the stabilization phase.
Statistical analyses
This post hoc analysis included patients who received at least 1 dose of brexpiprazole during the conversion phase. Patients were stratified into four “conversion groups” according to the amount of time spent in the conversion phase: 1–7 days, 8–14 days, 15–21 days, and 22–33 days (up to 33 days rather than 28 days to allow for minor variations in visit timings). Patients in each group were followed for up to 8 weeks from the start of the conversion phase (their first dose of brexpiprazole). Eight weeks of brexpiprazole treatment was chosen so that patients in each conversion group had the same exposure to brexpiprazole, and with sufficient time to assess efficacy. Depending on the amount of time spent in the conversion phase, 8 weeks of brexpiprazole treatment comprised 1–4 weeks in the conversion phase and 4–7 weeks in the stabilization phase (Figure 1).
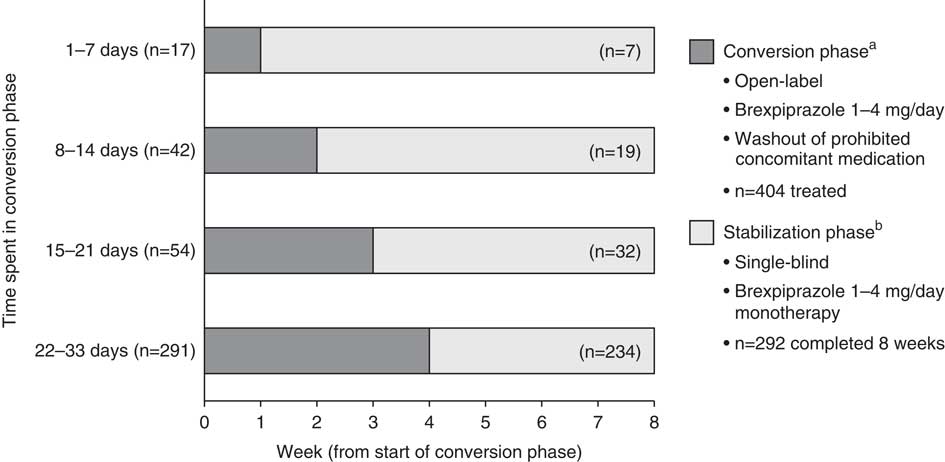
Figure 1 Definition of conversion groups, with patient flow through the conversion and stabilization phases. Data for patients who received at least 1 dose of brexpiprazole in the conversion phase. aWeekly visits; bweekly visits for 4 weeks, every two weeks thereafter.
The four conversion groups were compared in terms of time to discontinuation for different reasons within the first 8 weeks of brexpiprazole treatment, plotted as Kaplan–Meier survival analysis curves. This analysis was performed to identify any differences in discontinuation rates according to the speed of cross-titration from a prior antipsychotic to brexpiprazole.
Next, the conversion groups were further split, as follows: (1) patients who converted to brexpiprazole (i.e., completed the conversion phase) and also completed 8 weeks of brexpiprazole treatment, (2) patients who converted to brexpiprazole but discontinued in <8 weeks, and (3) patients who discontinued prior to converting to brexpiprazole (i.e., discontinued during the conversion phase). These various subgroups were generated in order to identify the differences between patients who successfully converted to brexpiprazole and those who did not, in terms of reason for discontinuation, baseline demographic and clinical characteristics, type of baseline concomitant antipsychotic, and the incidence of TEAEs. Thus, these analyses were performed to assess the generalizability of the findings, to identify risk factors for early discontinuation, and to identify characteristics that may be less represented in the analyzed sample.
Finally, in the group of patients who converted to brexpiprazole and completed 8 weeks of brexpiprazole treatment, the change from baseline in PANSS total score and CGI-S score were calculated for each conversion group.
In general, descriptive statistics are presented; due to the differences in sample size between groups, further statistical analysis was considered inappropriate. A chi-squared test was used to assess differences in baseline concomitant antipsychotic use between groups. One-way analysis of variance (ANOVA) was used to compare changes from baseline in PANSS total and CGI-S scores between conversion groups.
Results
Patient disposition
A total of 404 patients received at least 1 dose of brexpiprazole during the conversion phase and were included in this analysis. All patients were currently receiving at least one antipsychotic other than brexpiprazole at baseline, and, therefore, no patients entered the conversion phase based on prohibited concomitant medications alone.
Of the 404 patients treated with brexpiprazole (Figure 1), the majority (72.0%) spent 22–33 days in the conversion phase. Only a small number of patients spent 1–7 days (4.2%), 8–14 days (10.4%), or 15–21 days (13.4%) in the conversion phase.
Times to discontinuation from the start of the conversion phase to 8 weeks are presented in Figure 2: (a) due to all reasons other than the sponsor terminated the study (in this maintenance study, efficacy [as measured by the time from randomization to impending relapse] was demonstrated at a prespecified interim analysis [conducted after 45 events of impending relapse], and so the trial was terminated early);Reference Fleischhacker, Hobart and Ouyang13 (b) due to lack of efficacy; and (c) due to adverse events. Overall, discontinuation rates due to lack of efficacy and due to adverse events were low, regardless of time spent in the conversion phase.
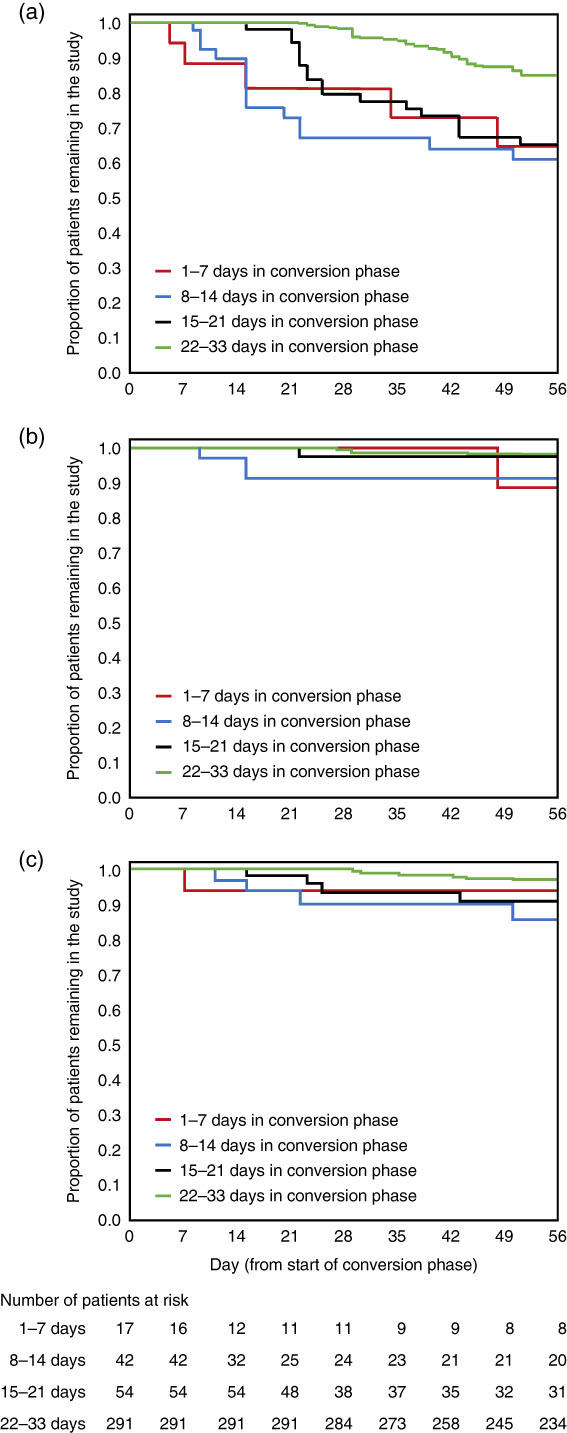
Figure 2 Kaplan–Meier curves, by conversion group, of time to discontinuation during the first 8 weeks of brexpiprazole treatment (a) due to all reasons other than sponsor terminated study; (b) due to lack of efficacy; and (c) due to adverse events. Data for patients who received at least 1 dose of brexpiprazole in the conversion phase.
In total, 292 (72.3%) of the 404 patients were converted to brexpiprazole and completed 8 weeks. Fifty-four patients (13.4%) were converted to brexpiprazole but discontinued in <8 weeks, and 58 patients (14.4%) discontinued prior to converting to brexpiprazole. Of the 292 patients who completed 8 weeks of brexpiprazole treatment, most were converted to brexpiprazole over 22–33 days (80.1%), and fewer were converted over 1–7 days (2.4%), 8–14 days (6.5%), or 15–21 days (11.0%).
Reasons for discontinuation are given in Table 2. For patients who discontinued in <8 weeks, whether they converted to brexpiprazole or discontinued prior to converting, the most common reasons for discontinuation were that the patient withdrew consent and that the sponsor terminated the study early due to the positive results of the interim analysis.
Table 2 Reasons for brexpiprazole treatment discontinuation, by conversion group, for patients who converted to brexpiprazole but discontinued in <8 weeks, and for patients who discontinued prior to converting to brexpiprazolea
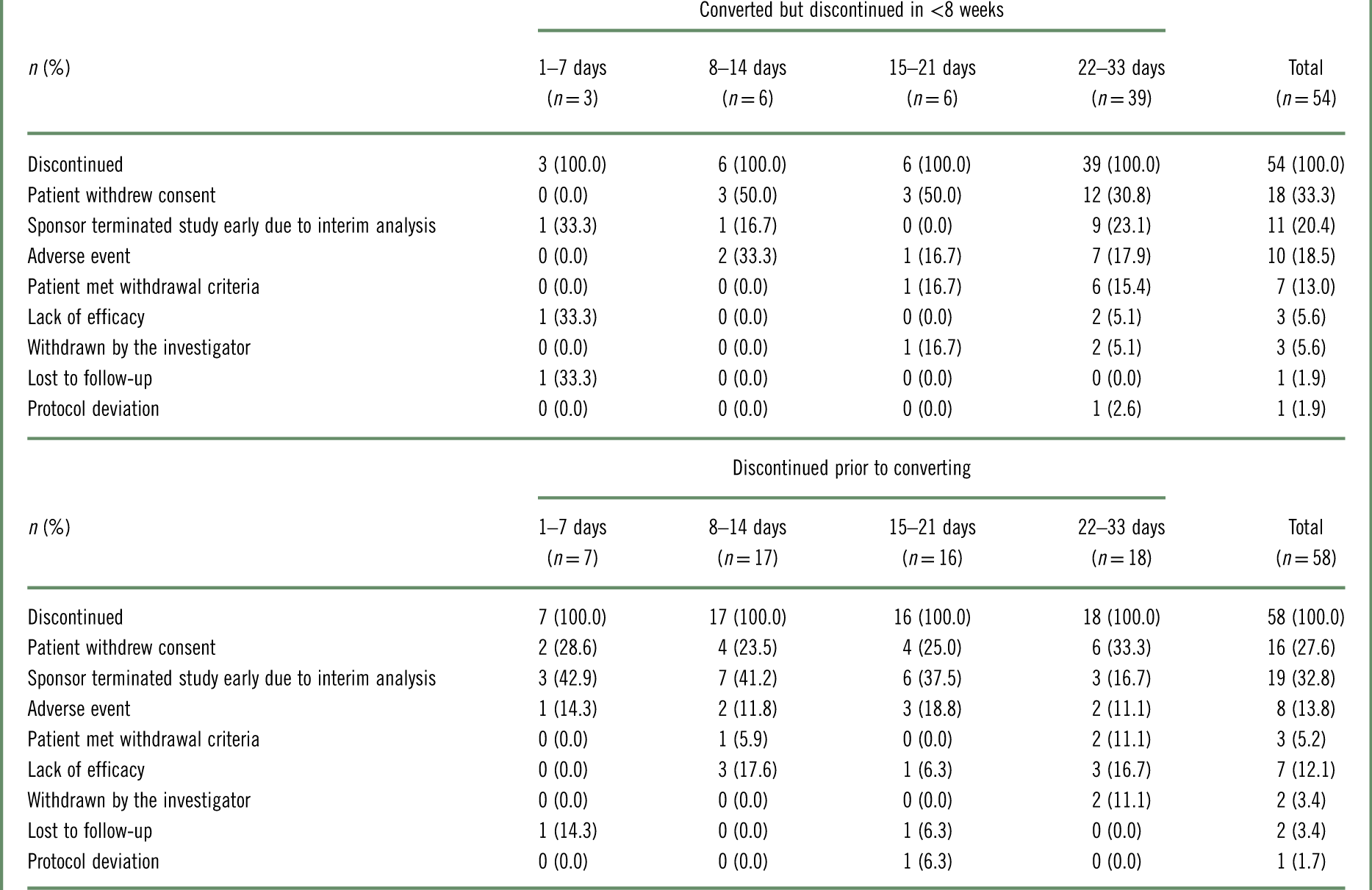
a Data for patients who received at least 1 dose of brexpiprazole in the conversion phase.
Patient characteristics
As shown in Table 3, baseline demographics were comparable between patients who converted to brexpiprazole and completed 8 weeks and patients who discontinued in <8 weeks (converted or nonconverted). Regarding clinical characteristics, patients who discontinued in <8 weeks had a higher mean PANSS total score at baseline than patients who converted and completed 8 weeks (85.2 vs. 81.2).
Table 3 Baseline demographic and clinical characteristics, by conversion groupa
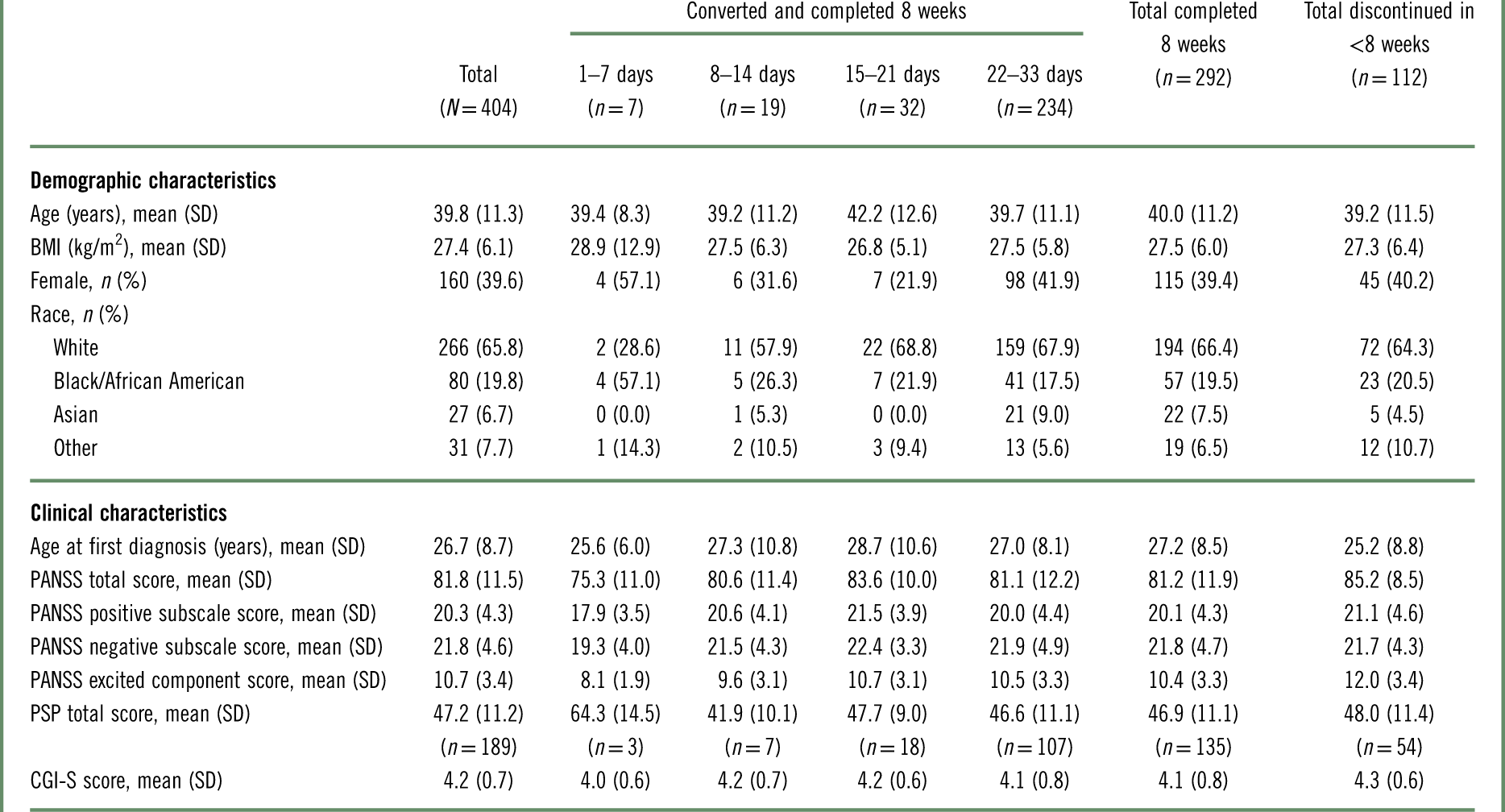
BMI=body mass index; CGI-S=Clinical Global Impressions–Severity of illness; PANSS=Positive and Negative Syndrome Scale; PSP=Personal and Social Performance scale; SD=standard deviation.
a Data for patients who received at least 1 dose of brexpiprazole in the conversion phase.
Among patients who converted and completed 8 weeks, baseline demographic and clinical characteristics showed a number of inconsistencies between conversion groups, which should be considered in the context of the low number of patients in the 1–7 days (n=7) and 8–14 days (n=19) groups (Table 3). For example, the proportions of patients who were female or who were black were higher in the 1–7 day group (57.1% and 57.1%, respectively) than in the other conversion groups (21.9–41.9% and 17.5–26.3%, respectively). Furthermore, the 1–7 day group had less severe disease than the other conversion groups, as measured by PANSS total and Personal and Social Performance (PSP) scale total scores.
There were no notable differences in patient characteristics between conversion groups for patients who converted and completed 8 weeks and patients who discontinued in <8 weeks (data not shown).
Baseline concomitant antipsychotics by conversion group are listed in Table 4. There were no statistically significant differences between patients who converted to brexpiprazole and completed 8 weeks and patients who discontinued in <8 weeks (converted or nonconverted), in terms of the proportion of patients who switched from antipsychotics with relatively strong antihistaminergic/anticholinergic or antidopaminergic properties or long-acting injectable (LAI) antipsychotics.
Table 4 Concomitant antipsychotics from which patients were switched to brexpiprazole during the conversion phase, by conversion groupa

a Data for patients who received at least 1 dose of brexpiprazole in the conversion phase. Patients may have received more than one concomitant antipsychotic. Antipsychotics are grouped according to their pharmacodynamic properties, as previously describedReference Correll8 or as defined by the authors.
b p values were calculated using a chi-squared test.
c Quetiapine or quetiapine fumarate.
d Trifluoperazine or trifluoperazine hydrochloride.
e Zuclopenthixol, zuclopenthixol acetate, or zuclopenthixol hydrochloride.
f Chlorprothixene or chlorprothixene hydrochloride.
g Fluphenazine or fluphenazine hydrochloride.
Among patients who converted and completed 8 weeks, there were no statistically significant differences between conversion groups in terms of the proportion of patients who switched from antipsychotics with relatively strong antihistaminergic/anticholinergic or antidopaminergic properties (Table 4). Similar nonstatistically significant differences between conversion groups were observed for patients who discontinued in <8 weeks (data not shown).
TEAEs
The incidence of TEAEs was comparable between the patients who converted to brexpiprazole and completed 8 weeks (49.7%) and the patients who discontinued in <8 weeks (converted or nonconverted) (47.3%), although the latter group, by definition, spent less time on brexpiprazole.
TEAEs with incidence≥2.0% during the first 8 weeks of brexpiprazole treatment are listed in Table 5 and Supplementary Table 1. Across all patients, the most common individual TEAEs (incidence≥5%) were insomnia (11.1%), headache (7.2%), agitation (6.2%), and akathisia (5.2%).
Table 5 Treatment-emergent adverse events (TEAEs) in the 8 weeks from the start of the conversion phase (the first dose of brexpiprazole), by conversion groupa
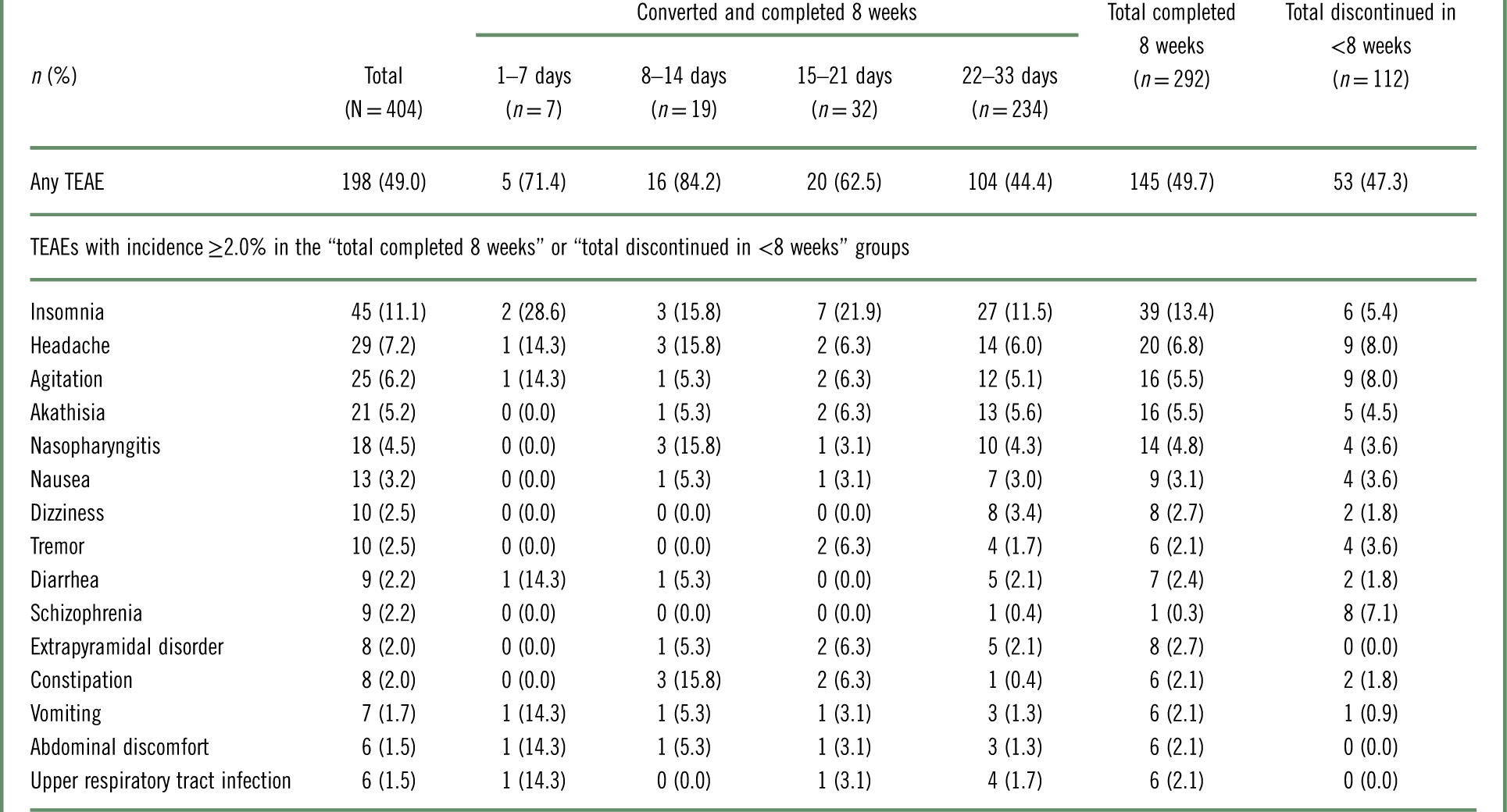
a Data for patients who received at least 1 dose of brexpiprazole in the conversion phase.
Among patients who converted and completed 8 weeks, the incidence of TEAEs over 8 weeks was lower among those converted over 22–33 days (44.4%) than in the other conversion groups (62.5–84.2%).
Efficacy
Changes from baseline in PANSS total and CGI-S scores are presented in Table 6. Among patients who converted to brexpiprazole and completed 8 weeks, each conversion group showed improvements in PANSS total and CGI-S scores from baseline to stabilization phase weeks 4, 6, and 8. There were no statistically significant differences in the magnitude of improvement from baseline between the conversion groups.
Table 6 Change from baseline in PANSS total score and CGI-S score with brexpiprazole treatment, by conversion groupa
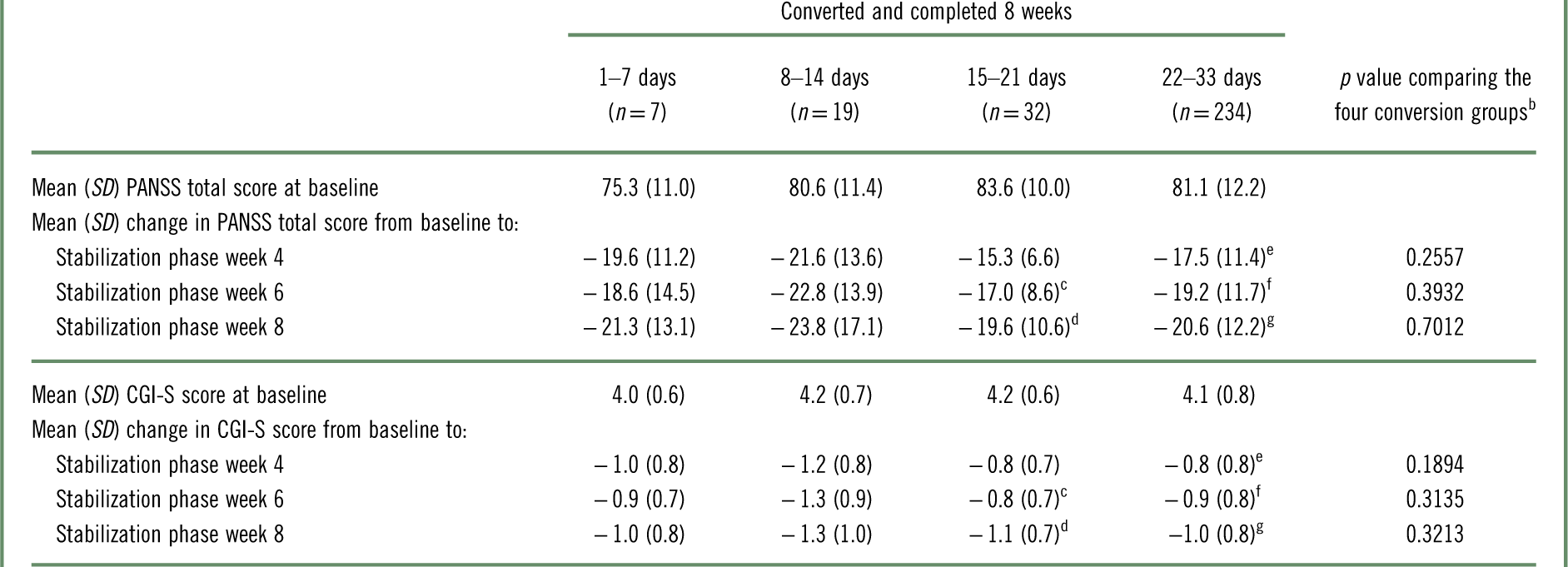
CGI-S=Clinical Global Impressions–Severity of illness; PANSS=Positive and Negative Syndrome Scale; SD=standard deviation.
a Data for patients who received at least 8 weeks of brexpiprazole treatment.
b p values were calculated using one-way analysis of variance.
c n=30.
d n=27.
e n=226.
f n=215.
g n=199.
Discussion
In this post hoc analysis of a long-term, controlled clinical study of brexpiprazole as maintenance treatment for schizophrenia, patients were converted from previous antipsychotic(s) to brexpiprazole monotherapy over a time period of 1–4 weeks, according to the investigators’ judgment (and the protocol-defined washout of prohibited concomitant medications). The majority of patients who were successfully switched and completed 8 weeks of brexpiprazole treatment were converted from previous antipsychotic(s) to brexpiprazole monotherapy over a period of 22–33 days. Regardless of the conversion schedule, the rates of discontinuation due to lack of efficacy or due to adverse events were low and showed only small differences. In other words, groups of patients with whom clinicians attempted conversion over shorter time periods were no more likely to discontinue than groups for whom conversion was attempted over longer time periods. It is unclear if clinicians used unmeasured patient characteristics to preselect patients in whom they attempted a shorter conversion schedule during the switch to brexpiprazole. Similarly, it remains unclear whether clinicians chose the conversion schedules a priori, or if they prolonged the conversion period in patients who experienced emergent symptoms or problems. There was a numerically higher incidence of TEAEs over 8 weeks among patients who converted to brexpiprazole over 1–7 days and 8–14 days than among those who converted over 22–33 days, but the low number of patients in these groups limits between-group interpretations.
Oral antipsychotics were cross-titrated over 1–4 weeks and, within the defined 1–4 week window, clinicians used their judgment regarding the up-titration of brexpiprazole and the down-titration of previous antipsychotic: there was no defined schedule for dose escalation of brexpiprazole, delay prior to discontinuation of previous antipsychotic, or rate of discontinuation of previous antipsychotic. LAI antipsychotics were stopped abruptly prior to brexpiprazole initiation, which, due to the long half-lives of LAIs, can be equated to cross-titration.Reference Peuskens18, Reference Correll, Citrome and Haddad19 Antipsychotic switching may be complicated by potential pharmacodynamic and pharmacokinetic withdrawal and rebound phenomena when switching from a highly antihistaminergic/anticholinergic antipsychotic to a less antihistaminergic/anticholinergic antipsychotic, to a partial D2 agonist from a full D2 antagonist, or to an antipsychotic with a longer half-life from one with a shorter half-life.Reference Buckley and Correll2, Reference Correll8 Because brexpiprazole is a D2 partial agonist with minimal antihistaminergic/anticholinergic properties, investigators may have chosen longer conversion periods to avoid these potential withdrawal and rebound phenomena.Reference Correll8, Reference Maeda, Sugino and Akazawa10 In general, switching strategies with overlap between the two agents may be the most beneficial to avoid intra-switch worsening/complications. In clinical practice, however, a longitudinal study in the Netherlands showed that the majority of antipsychotics are switched abruptly.Reference de Smidt, Haffmans and Hoencamp20 Furthermore, an open-label study of switching to aripiprazole (another D2 partial agonist) from other antipsychotics found no safety differences between abrupt switching and cross-titration.Reference Casey, Carson and Saha21
Prior studies have not always shown a difference between switching strategies,Reference Takeuchi, Thiyanavadivel, Agid and Remington9, Reference Takeuchi, Kantor and Uchida22, Reference Takeuchi, Thiyanavadivel, Agid and Remington23 for reasons that may include the hospitalization status of patients, benzodiazepine use (often permitted but unmeasured) that could counter rebound phenomena, and the differing pharmacologic profiles of pre-switch antipsychotics. A meta-analysis of five randomized controlled studies comparing plateau cross-titration with nondelayed cross-titration in patients with schizophrenia found no statistically significant differences on any clinical outcomes, including discontinuation rate, psychopathology, extrapyramidal symptoms, and TEAEs.Reference Takeuchi, Thiyanavadivel, Agid and Remington9 The individual randomized controlled trials included in the meta-analysis also failed to show the superiority of either switching strategy.Reference Takeuchi, Thiyanavadivel, Agid and Remington9 However, in the meta-analysis, data for all antipsychotics were pooled together, independent of their pharmacokinetic and pharmacodynamic properties, and the different pharmacologic properties of the baseline, pre-switch antipsychotics were similarly not considered, which may have obscured differences across switching strategies. While not reflecting real-world clinical practice, these inconclusive findings seem to indicate that it may be difficult to generalize switching approaches (each patient may require an individualized switching strategy) and that switching strategies may especially differ for switches between antipsychotics with similar versus different pharmacologic properties.Reference Correll8 The current meta-analyses on switching did not have sufficient power to adequately address this issue, and future studies are needed for clarification, particularly now that three different partial D2 agonists are available for which switches from full D2 antagonists would at least theoretically be complicated by an interaction between partial D2 agonism and an upregulated D2 receptor system.Reference Correll8
In the present analysis, the efficacy of brexpiprazole was similar in all conversion groups, as demonstrated by improvements from baseline to stabilization phase weeks 4, 6, and 8 in PANSS total and CGI-S scores. Because patients were cross-titrated from a wide variety of antipsychotic drugs, the improvements in PANSS total and CGI-S indicate that conversion to brexpiprazole may be efficacious for acutely ill patients (who received at least 8 weeks of brexpiprazole treatment) regardless of their prior therapy or the switching schedule.
Limitations of the present analysis include: (1) that the switching strategy/duration of overlap was the clinicians’ choice and not randomized, yet this feature also increases the real-world applicability and generalizability of the findings; (2) the exploratory, post hoc nature of the analyses; (3) the low numbers of patients who were converted to brexpiprazole over 1–7 and 8–14 days, which mandates caution when interpreting the findings that improvements in efficacy appeared to be irrespective of conversion rate; (4) the multiplicity of baseline antipsychotics, precluding a more definitive assessment beyond pharmacologically similar subgroups of antipsychotics as to whether the switch from any specific antipsychotic with specific pharmacodynamic and pharmacokinetic properties would be more or less problematic/associated with withdrawal or rebound phenomena when switching to brexpiprazole; and (5) the lack of information from clinicians about the reasons for the switching strategy employed. Strengths of the study include the overall size of the study population; the structured study phases and assessments; and the real-world decision-making regarding the switching strategy employed (within the protocol-specified 1–4 week duration).
Conclusions
The results presented here provide a mostly clinically driven evidence base from which clinicians can choose a switching paradigm for patients initiating brexpiprazole that best meets their patients’ needs. In general, for a successful switch entailing at least 8 weeks of brexpiprazole treatment, a longer switching period of 22–33 days was employed and was efficacious. Additional, real-world data on preferred and successful switching strategies could be helpful to inform clinicians’ optimal use of brexpiprazole in patient subgroups when converting treatment from specific other antipsychotics.
Acknowledgments
Funding for the primary study and for the writing of this article was provided by Otsuka Pharmaceutical Development & Commercialization Inc. (Princeton, New Jersey) and H. Lundbeck A/S (Valby, Denmark). Writing support was provided by Chris Watling, PhD, assisted by his colleagues at Cambridge Medical Communication Ltd (Cambridge, UK).
Disclosures
Christoph U. Correll has been a consultant and/or advisor to or has received honoraria from: Alkermes, Allergan, Bristol-Myers Squibb, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, LB Pharma, Lundbeck, Medavante, Medscape, Neurocrine, Otsuka, Pfizer, Sunovion, Takeda, and Teva. He has provided expert testimony for Bristol-Myers Squibb, Janssen, and Otsuka. He has served on a data safety monitoring board for Lundbeck, and Pfizer. He has received grant support from Takeda.
Lily Shi, Catherine Weiss, Mary Hobart, Ruth A. Duffy, and Ross A. Baker are full-time employees of Otsuka Pharmaceutical Development & Commercialization Inc.
Anna Eramo is a full-time employee of Lundbeck LLC.
Emmanuelle Weiller was a full-time employee of H. Lundbeck A/S at the time of this work.
Supplementary materials
To view supplementary material for this article, please visit https://doi.org/10.1017/S1092852918001086