CVD, which is often associated with hyperlipidaemia, has become the major cause of death in many countries( Reference Hyson, Rutledge and Berglund 1 , Reference Kłosiewicz-Latoszek, Pachocka and Górska 2 ). Elevated peripheral NEFA and de novo lipogenesis predominantly contribute to the accumulation of lipids in the liver( Reference Donnelly, Smith and Schwarzenberg 3 ), which is mostly controlled by the intake of different dietary fats through changes in hepatic enzyme activities related to the metabolism of fatty acids( Reference Iritani, Fukuda and Tada 4 ). In general, fat accumulation in the body may be considered as the net result of the balance among dietary absorbed fat, endogenous synthesis of fat and the catabolism of fat via β-oxidation.
It has been shown that different fatty acids exert different effects on the transcription of specific genes involved in hepatic lipid metabolism( Reference Clarke 5 , Reference Jump, Botolin and Wang 6 ). Transcriptional regulation of fatty acid metabolism is considered to be the major regulatory mechanism controlling lipid homeostasis, which is executed by a variety of transcription factors( Reference Guillou, Martin and Pineau 7 ). Sterol response element-binding protein-1c (SREBP-1c; 68 kDa) is a transcription factor known to be the major regulator of lipogenic enzymes( Reference Horton, Goldstein and Brown 8 ) and reduced expression of SREBP-1c is a primary mechanism by which dietary fatty acids reduce hepatic lipogenesis. In contrast with the numerous factors involved in fatty acid synthesis and storage, the transcriptional regulation of fatty acid oxidation has long been dominated by PPARα, which is a nuclear receptor activated by natural ligands such as fatty acids and stimulates β-oxidation of SFA in mitochondria and PUFA in the peroxisomes( Reference Latruffe, Cherkaoui Malki and Nicolas-Frances 9 ). Some of the enzymes of these two systems are highly inducible by natural and synthetic ligands of PPARα( Reference Reddy and Hashimoto 10 , Reference Hashimotto, Cook and Qi 11 ).
Nutritional status, particularly the dietary fatty acid pattern of the individual, plays a key role in regulating fatty acid metabolism( Reference Kelley, Nelson and Hunt 12 ). The diversity in fatty acid structure resulting from differences in chain length, degree of unsaturation, position and stereoisomeric configuration of the double bonds may affect the rate of fatty acid oxidation. Although the usual dietary fats composed of mainly long-chain fatty acids are known to be powerful inhibitors of lipogenesis in the liver and adipose tissue( Reference Leveille 13 ), paradoxically they produce obesity( Reference Mickelsen, Takahashi and Craig 14 ).
Coconut oil extracted from dried copra is the characteristic cooking medium in most of the culinary preparations in all coconut-producing countries. Nowadays, virgin coconut oil (VCO) extracted from fresh coconut kernel by wet processing is becoming very valuable because of its biological properties. Individual fatty acid analysis of the two types of coconut oil has revealed the presence of higher amounts of short- and medium-chain TAG, mainly lauric acid( Reference Nevin and Rajamohan 15 ). The major advantage of medium-chain fatty acids in coconut oil is that they are more rapidly oxidised by both mitochondrial and peroxisomal pathways compared with MUFA and PUFA( Reference DeLany, Windhauser and Champagne 16 ). Moreover, VCO extraction by wet processing retains higher amounts of unsaponifiable components such as polyphenols, tocopherols and phytosterols( Reference Nevin and Rajamohan 17 , Reference Nevin and Rajamohan 18 ). Previous studies have reported that VCO possesses significant hypolipidaemic, antioxidant and anti-thrombotic effects compared with copra oil (CO)( Reference Nevin and Rajamohan 15 , Reference Nevin and Rajamohan 17 , Reference Nevin and Rajamohan 19 ). In addition, our recent reports have revealed that VCO has beneficial effects on regulating hepatic lipid metabolism in vivo ( Reference Arunima and Rajamohan 20 ).
In view of these findings, the present study was carried out to evaluate the effects of VCO compared with CO, oleate-rich olive oil (OO) and linoleate-rich sunflower-seed oil (SFO) on the synthesis and oxidation of fatty acids and its effect on the transcriptional regulation of fatty acid metabolism in rats.
Materials and methods
Chemicals
Acetyl CoA, malonyl CoA, NAD phosphate, l-carnitine, ATP, TRI reagent and primers used for RT-PCR were obtained from Sigma Chemicals. cDNA synthesis and PCR reactions were performed using a kit purchased from Fermentas, Thermo Fisher Scientific Inc. and all other chemicals used were of the highest analytical grade.
Extraction of virgin coconut oil and copra oil
The solid endosperm of mature coconut (West coast tall variety) was crushed, made into viscous slurry and squeezed through cheesecloth to obtain coconut milk, which was refrigerated for 48 h, then subjected to mild heating (50°C) in a thermostat oven. The obtained VCO filtered through cheesecloth was used for the present study( Reference Nevin and Rajamohan 17 ). CO was extracted from coconut meat, which was dried in sunlight continuously for 4 d to remove moisture and the resulting copra was pressed in a mill to obtain CO( Reference Nevin and Rajamohan 17 ).
Olive oil and sunflower-seed oil
OO (Pietro Coricelli brand) and SFO (Sun drop brand) were purchased from a local market.
Animals
Male Sprague–Dawley rats (100–120 g) bred in our departmental animal house were used for the study. Animals were individually housed under hygienic conditions in polypropylene cages in a room maintained at an ambient temperature of 25 ± 1°C with a 12 h light–12 h dark cycle. The entire protocol was approved by the Animal Ethics Committee, University of Kerala (IAEC-KU-8/09-10-BC-TR [17]) and the animals were handled using welfare guidelines for laboratory animals.
Experimental design
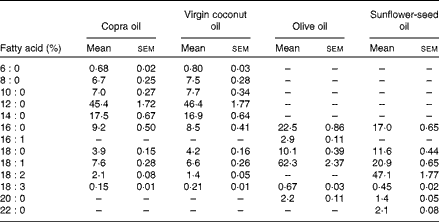
A total of twenty-four rats were divided into four groups. The experimental design was as follows: group I rats were given 8 % CO; group II rats were given 8 % VCO; group III rats were given 8 % OO; and group IV rats were given 8 % SFO. Each rat was given 12 g synthetic diet containing 8 % dietary oils daily for 45 d as described previously (Table 1)( Reference Nevin and Rajamohan 17 , Reference Lombardo, Chicco and D'Alessandro 21 ). The fatty acid compositions of test oils were estimated by GC-MS and reported previously (Table 2)( Reference Arunima and Rajamohan 22 ). Food intakes of rats were noted daily and the body weight was determined weekly. After 45 d, animals were fasted overnight and killed by sodium pentathone injection. Blood and tissues were collected for various estimations.
Table 1 Formulation of synthetic diet used for the study
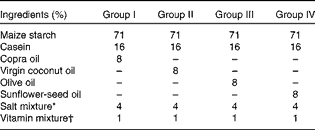
* Composition of salt mixture (per kg diet)( Reference Hubbell, Mendel and Wakeman 71 ): NaCl, 105 mg; KCl, 120 mg; KH2PO4, 310 mg; Ca4(PO4)2, 149 mg; CaCO3, 210 mg; MnSO4, 0·2 mg; KAl(SO4)2, 0·09 mg; MgSO4, 90 mg; FeSO4·7H2O, 14·7 mg; CuSO4, 0·37 mg; NaF, 0·57 mg; KI, 0·05 mg; ZnCl2, 0·02 mg; CoCl2, 0·15 mg.
† Composition of vitamin mixture (per 100 g diet): retinyl palmitate, 3 mg ergocalciferol, 3·75 μg; α-tocopherol, 12 mg; menadione, 0·3 mg; thiamine, 1 mg; riboflavin, 1 mg; pyridoxine, 0·6 mg; niacin, 10 mg; calcium pantothenate, 5 mg; inositol, 20 mg; folic acid, 0·4 mg; vitamin B12, 3 μg; biotin, 20 μg; p-aminobenzoic acid, 5 mg; choline chloride, 300 mg.
Table 2 Fatty acid composition of test oils in the diet (Mean values of three estimations with their standard errors)
Change in body weight
The body weight of each animal was noted before starting the feeding experiment and also on the experimental day. Change in body weight was calculated as follows:
Lipid analysis
Total lipids from serum and tissues were extracted using chloroform–methanol (2:1, v/v) as described previously( Reference Folch, Lees and Sloane Stanley 23 ). Tissue (500 mg) or serum (0·5 ml) was homogenised with chloroform–methanol (2:1, v/v), filtered and washed with chloroform–methanol at least three times. Calcium chloride (0·02 %) was added to the mixture (20 % of the total volume of the extract), mixed vigorously and allowed to stand for a few minutes. The upper layer was washed three times with 5 ml chloroform–methanol–calcium chloride (3:48:48, by vol.). The washed upper layer was evaporated to dryness and the residue was redissolved in a known volume of chloroform. From this, samples were used for lipid analysis. Total cholesterol was estimated as described by Carr & Drekter( Reference Carr and Drekter 24 ). TAG were estimated by the method of Van Handel & Zilversmit( Reference Van Handel and Zilversmit 25 ).
Estimation of phospholipids
Phospholipids were estimated by the method of Zilversmit & Davis( Reference Zilversmit and Davis 26 ). A sample of the extract was pipetted out into Kjeldahl flask and evaporated to dryness. Then 1 ml of H2SO4 (2·5 m) was added and the sample was digested until it became light brown. It was cooled to room temperature and two drops of HNO3 (2 m) were added and the sample digested again until it became colourless. The Kjeldahl flask was cooled, 1 ml water was added and heated in a boiling water-bath for 5 min. Then 1 ml of ammonium molybdate (2·5 %) and 0·1 ml of 1-amino 2-naphthol 4-sulfonic acid (ANSA) reagent were added and the volume made up to 10 ml with distilled water. After 10 min, absorbance was measured at 660 nm using a Shimadzu 1240 spectrophotometer.
Isolation of mitochondria and peroxisomes
Mitochondria were separated from the heart according to Tyler & Gonze( Reference Tyler and Gonze 27 ). Rat heart tissue was washed in cold MSE medium (0·225 m-mannitol, 0·075 m-sucrose and 0·05 mm-EDTA, pH 7·4) and finely chopped. The chopped heart tissue was washed continuously with MSE medium to remove adhering blood. The heart tissue was then homogenised in 5 ml of MSE medium and 0·05 ml of un-neutralised Tris buffer (1 m). The homogenate was then diluted to 40 ml with MSE medium, divided equally into two centrifuge tubes and centrifuged at 8000 g for 10 min. The supernatant fraction was decanted and the whole pellet was re-suspended in 40 ml of MSE medium. After a second centrifugation at 700 g for 10 min, the supernatant fraction was collected and the pellets were discarded. A third centrifugation at 8000 g for 10 min was done to obtain mitochondrial fractions and the supernatant fraction was discarded. The mitochondrial pellet was then re-suspended in the appropriate buffer as described for each enzyme estimation. The 700 g peroxisomal fraction obtained during mitochondrial isolation was used for studying peroxisomal β-oxidation( Reference Grum, Hansen and Drackley 28 ). The protein contents in the subcellular fractions were determined by the method of Lowry et al. ( Reference Lowry, Rosebrough and Farr 29 ).
Mitochondrial and peroxisomal β-oxidation
Mitochondrial β-oxidation( Reference Osmundsen 30 ) and the activities of carnitine palmitoyl transferase I (CPT I)( Reference Ide, Watanabe and Sugano 31 ), acyl CoA dehydrogenase (ACDH)( Reference Dommes and Kunau 32 ), enoyl CoA hydratase( Reference Osumi and Hashimoto 33 ), β-hydroxy acyl CoA dehydrogenase( Reference Osumi and Hashimoto 34 ) and 3-ketoacyl CoA thiolase( Reference Lynen and Ochoa 35 ) were determined in isolated mitochondria. The peroxisomal fraction was used for determining the activity of acyl CoA oxidase (ACO)( Reference Shimizu, Yasui and Tani 36 ).
De novo fatty acid synthesis
The enzymes involved in de novo fatty acid synthesis, namely acetyl CoA carboxylase (ACC)( Reference Inoue and Lowenstein 37 ) and fatty acid synthase (FAS)( Reference Kim, Neudahl and Deal 38 ), were assayed in liver using a Shimadzu 1240 spectrophotometer.
Isolation of total genomic RNA
Total genomic RNA was isolated from the heart using TRI reagent (Sigma Aldrich) by the method described by Chomczynski & Mackey( Reference Chomczynski and Mackey 39 ).
cDNA synthesis and RT-PCR
cDNA synthesis and PCR reactions were performed in a thermocycler (Eppendorf AG) according to the manufacturer's instructions using a kit purchased from Fermentas. The primers used were: FAS (forward) 5′-AGG TGC TAG AGG CCC TGC TA-3′ and (reverse) 5′-GTG CAC AGA CAC CTT CCC AT-3′; SREBP-1c (forward) 5′-GGAGCCATGGATTGCACATT-3′ and (reverse) 5′-AGGAAGGCTTCCAGAGAGGA-3′; CPT I (forward) 5′-GGA GAC AGA CAC CAT CCA ACA TA-3′ and (reverse) 5′-AGG TGA TGG ACT TGT CAA ACC-3′; ACO (forward) 5′-CTT TCT TGC TTG CCT TCC TTC TCC-3′ and (reverse) 5′-GCC GTT TCA CCG CCT CGT A-3′; PPARα (forward) 5′-TCACACAATGCAATCCGTTT-3′ and (reverse) 5′-GGCCTTGACCTTGTTCATGT-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward) 5′-CCT TCA TTG ACC TCA ACT AC-3′ and (reverse) 5′-GGA AGG CCA TGC CAG TGA GC-3′. The PCR products were electrophoresed on 1·5 % agarose gel and visualised by staining with ethidium bromide. The gels were subjected to densitometric scanning (Bio-Rad Gel Doc) to determine the optical density of each and then normalised against an internal control (GAPDH) using Quantity One imaging software (Bio-Rad Laboratories).
Statistical analysis
Data are presented as means with their standard errors. Statistical analysis was performed by one-way ANOVA followed by Duncan's multiple-range tests using the SPSS/PC (version 17.0; SPSS) software package program. P values < 0·05 were considered significant.
Results
Dietary intake and change in body weight
There was no significant difference in diet consumption and change in body weight among the experimental groups (Table 3).
Table 3 Food intake and change in body weight (Mean values of six rats per group with their standard errors)
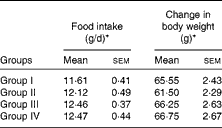
Group I, 8 % copra oil-fed rats; group II, 8 % virgin coconut oil-fed rats; group III, 8 % olive oil-fed rats; group IV, 8 % sunflower-seed oil-fed rats.
* Mean values were not significantly different.
Effect on serum and liver lipids
Table 4 summarises the levels of lipids in the serum and liver of rats fed the different test oils. The concentration of total cholesterol (1·87 mmol/l serum; 128·57 mg/100 g liver), TAG (0·11 mmol/l serum; 154·78 mg/100 g liver) and phospholipids (1·01 mmol/l serum; 1788 mg/100 g liver) were decreased significantly (P< 0·05) in VCO-fed rats compared with those fed CO, OO and SFO. Supplementation of VCO decreased the cholesterol levels (22·72 % decrease in serum and 51·25 % decrease in liver) compared with rats fed CO. Similarly there was a 28·62 % decrease in serum cholesterol levels and a 54·05 % decrease in hepatic cholesterol levels in VCO-fed rats compared with rats fed OO. Decreased levels of TAG (15·38 % decrease in serum and 15·13 % decrease in liver) and phospholipids (40·93 % decrease in serum and 18·34 % decrease in liver) were observed in VCO-fed rats compared with rats fed CO. A significant increase in TAG and phospholipids levels was observed in OO- (26·66 % increase in serum TAG and 49·50 % increase in serum phospholipids) and SFO- (21·42 % increase in serum TAG and 41·28 % increase in serum phospholipids) compared with VCO-fed rats.
Table 4 Cholesterol, TAG and phospholipid concentrations in serum and liver (Mean values of six rats per group with their standard errors)
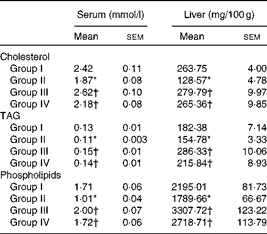
Group I, 8 % copra oil-fed rats; group II, 8 % virgin coconut oil-fed rats; group III, 8 % olive oil-fed rats; group IV, 8 % sunflower-seed oil-fed rats.
* Mean value was significantly different from that of group I (P< 0·05).
† Mean value was significantly different from that of group II (P< 0·05).
Effect on synthesis and oxidation of fatty acids
Activities of enzymes involved in de novo fatty acid synthesis were assayed in the liver cytosolic fraction from rats fed the different test oils. Reduced rates of fatty acid synthesis were evident from the decreased activities of enzymes involved in fatty acid synthesis, namely ACC (9·38 U/mg protein) and FAS (18·78 U/mg protein), in VCO-fed rats compared with rats fed CO, OO and SFO (Fig. 1). However, the rates of mitochondrial β-oxidation (0·305 U/mg protein) and peroxisomal β-oxidation (4·53 U/mg protein) were significantly (P< 0·05) increased in rats fed VCO compared with the other oil-fed rats (Table 5). Significant decreases in the activities of CPT I and ACO were observed in rats fed CO (13·07 % decrease in CPT I and 18·86 % decrease in ACO), OO (29·08 % decrease in CPT I and 30·78 % decrease in ACO) and SFO (18·30 % decrease in CPT I and 27·78 % decrease in ACO) compared with VCO-fed rats (Fig. 2). The enzymes involved in mitochondrial β-oxidation, namely ACDH (0·768 U/mg protein), enoyl CoA hydratase (0·304 U/mg protein), β-hydroxy acyl CoA dehydrogenase (0·620 U/mg protein) and 3-ketoacyl CoA thiolase (0·224 U/mg protein), were also increased significantly (P< 0·05) in rats fed VCO compared with the other oil-fed groups (Table 6). Significant decreases in the activities of ACDH (67·83 %), ECDH (47·03 %), β-hydroxy acyl CoA dehydrogenase (65·64 %) and 3-ketoacyl CoA thiolase (39·73 %) were observed in OO-fed rats compared with VCO-fed rats (Table 6).

Fig. 1 Activity of acetyl CoA carboxylase (ACC) and fatty acid synthase (FAS) in copra oil- (□), virgin coconut oil- (![]() ), olive oil- (
), olive oil- (![]() ) and sunflower-seed oil- (
) and sunflower-seed oil- (![]() ) fed rats. Values are means of six rats per group, with their standard errors represented by vertical bars. *Mean value was significantly different from that of group I (P< 0·05). † Mean value was significantly different from that of group II (P< 0·05).
) fed rats. Values are means of six rats per group, with their standard errors represented by vertical bars. *Mean value was significantly different from that of group I (P< 0·05). † Mean value was significantly different from that of group II (P< 0·05).
Table 5 Effect of test oils on mitochondrial and peroxisomal β-oxidation (Mean values of six rats per group with their standard errors)
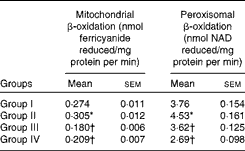
Group I, 8 % copra oil-fed rats; group II, 8 % virgin coconut oil-fed rats; group III, 8 % olive oil-fed rats; group IV, 8 % sunflower-seed oil-fed rats.
* Mean value was significantly different from that of group I (P< 0·05).
† Mean value was significantly different from that of group II (P< 0·05).

Fig. 2 Activity of carnitine palmitoyl transferase I (CPT I) and acyl CoA oxidase (ACO) in copra oil- (□), virgin coconut oil- (![]() ), olive oil- (
), olive oil- (![]() ) and sunflower-seed oil- (
) and sunflower-seed oil- (![]() ) fed rats. Values are means of six rats per group, with their standard errors represented by vertical bars. * Mean value was significantly different from that of group I (P< 0·05). † Mean value was significantly different from that of group II (P< 0·05).
) fed rats. Values are means of six rats per group, with their standard errors represented by vertical bars. * Mean value was significantly different from that of group I (P< 0·05). † Mean value was significantly different from that of group II (P< 0·05).
Table 6 Effect of test oils on mitochondrial β-oxidation enzymes (Mean values of six rats per group with their standard errors)

ACDH, acyl CoA dehydrogenase; ECHD, enoyl CoA hydratase; HOAD, β-hydroxy acyl CoA dehydrogenase; KAT, 3-ketoacyl CoA thiolase; group I, 8 % copra oil-fed rats; group II, 8 % virgin coconut oil-fed rats; group III, 8 % olive oil-fed rats; group IV, 8 % sunflower-seed oil-fed rats.
* Mean value was significantly different from that of group I (P< 0·05).
† Mean value was significantly different from that of group II (P< 0·05).
Effect on transcriptional regulation of fatty acid metabolism
There was a down-regulation in the mRNA expression of FAS (the multi-enzyme complex involved in de novo fatty acid synthesis) and its transcription factor SREBP-1c in VCO-fed rats compared with the other oil-fed groups (Fig. 3). On the other hand, the mRNA expression of PPARα and its target genes involved in fatty acid oxidation, namely CPT I and ACO, were up-regulated in VCO-fed rats compared with CO-, OO- and SFO-fed rats (Fig. 4).

Fig. 3 mRNA expression of fatty acid synthase (FAS) and sterol regulatory element-binding protein-1c (SREBP-1c) in copra oil- (CO; □), virgin coconut oil- (VCO; ![]() ), olive oil- (OO;
), olive oil- (OO; ![]() ) and sunflower-seed oil (SFO;
) and sunflower-seed oil (SFO; ![]() )-fed rats. Values are means of six rats per group, with their standard errors represented by vertical bars. * Mean value was significantly different from that of group I (P< 0·05). † Mean value was significantly different from that of group II (P< 0·05). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
)-fed rats. Values are means of six rats per group, with their standard errors represented by vertical bars. * Mean value was significantly different from that of group I (P< 0·05). † Mean value was significantly different from that of group II (P< 0·05). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.

Fig. 4 mRNA expression of PPARα and its target genes carnitine palmitoyl transferase I (CPT I) and acyl CoA oxidase (ACO) involved in fatty acid oxidation in copra oil- (CO; □), virgin coconut oil- (VCO; ![]() ), olive oil- (OO;
), olive oil- (OO; ![]() ) and sunflower-seed oil (SFO;
) and sunflower-seed oil (SFO; ![]() )-fed rats. Values are means of six rats per group, with their standard errors represented by vertical bars. * Mean value was significantly different from that of group I (P< 0·05). † Mean value was significantly different from that of group II (P< 0·05). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
)-fed rats. Values are means of six rats per group, with their standard errors represented by vertical bars. * Mean value was significantly different from that of group I (P< 0·05). † Mean value was significantly different from that of group II (P< 0·05). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Discussion
In the present study we compared the effects of VCO with CO, OO and SFO on the synthesis and oxidation of fatty acids and its effect on the molecular regulation of fatty acid metabolism in rats. There was no significant difference in food intake or change in body weight among the experimental groups. Supplementation of VCO decreased the concentration of lipids (cholesterol, TAG and phospholipids) in serum and liver when compared with CO-, OO- and SFO-fed rats. However, OO-fed rats showed an increased level of hepatic lipid accumulation compared with rats fed the other oils, which may be due to an increased lipogenesis and decreased catabolism of fats in OO-fed rats. There are reports that dietary fat influences the occurrences and prognosis of CHD, which is partially mediated by its effect on plasma lipids( Reference Hu, Manson and Willett 40 ).
Fatty acid biosynthesis in mammalian cells can be regulated in different ways. On a long-term basis, it is controlled by changes in the rate of synthesis of lipogenic enzymes( Reference Gibson, Lyons and Scott 41 ). As shown in Fig. 1, activities of enzymes involved in the de novo synthesis of fatty acids, namely ACC and FAS, were found to be decreased in VCO-fed rats compared with the other oil-fed rats. ACC catalyses the carboxylation of acetyl-CoA to produce malonyl-CoA for the biosynthesis of fatty acids( Reference Tong 42 ) and FAS is the multi-enzyme complex needed for the de novo synthesis of fatty acids. There are reports of increased activities of lipogenic enzymes and hepatic lipid accumulation in OO-fed animals( Reference Portillo, Chavari and Duran 43 , Reference Takeuchi, Nakamoto and Mori 44 ). On the other hand, medium-chain TAG have a reductive effect on fat stores and a depressive effect on lipogenesis( Reference Lavau and Hashim 45 ). Fatty acid analysis of VCO extracted from fresh coconut kernel and CO extracted from dried copra revealed almost similar fatty acid content. Since there was no major difference in the fatty acid profiles among the two varieties of coconut oil, it is evident that the beneficial effect of VCO over CO is mainly due to the presence of unsaponifiable components, namely polyphenols and vitamin E. Studies revealed that apart from fatty acids, the unsaponifiable components present in dietary oils have a role in regulating hepatic lipid metabolism. Chemical analysis of the test oils has revealed that VCO by wet processing has increased polyphenolic contents (84 mg/100 g oil), significantly higher than CO (64·4 mg/100 g oil), OO (75·63 mg/100 g oil) and SFO (55·26 mg/100 g oil)( Reference Arunima and Rajamohan 20 ). HPLC analysis of the phenolic fraction of VCO has revealed the presence of increased amounts of caffeic acid (0·829 μg/100 g oil), p-coumaric acid (5·43 μg/100 g oil), ferulic acid (9·91 μg/100 g oil), (+)-catechin hydrate (0·983 μg/100 g oil) and syringic acid (2·51 mg/100 g oil) compared with CO, OO and SFO( Reference Arunima and Rajamohan 22 ); these observations are consistent with other studies( Reference Seneviratne and Dissanayake 46 ). In addition, the non-saponifiable fraction of VCO contains appreciably higher amounts of antioxidants, namely vitamin E (33·12 μg/100 g oil) and β-carotene (196 μg/100 g oil)( Reference Arunima and Rajamohan 20 , Reference Arunima and Rajamohan 22 ). Increased amounts of these non-saponifible components present in VCO may partly be responsible for the decreased lipogenesis and lipid accumulation compared with other oils. Increased amounts of these bioactive non-saponifiable components may partly be responsible for the decreased lipogenesis and lipid accumulation compared with other oils.
There are reports that polyphenols can inhibit hepatic lipogenesis, promote hepatic lipid clearance( Reference Yang, Peng and Chan 47 ) and decrease serum and hepatic lipid accumulation( Reference Zern, West and Fernandez 48 , Reference Fukuchi, Hiramitsu and Okada 49 ). Increased amounts of polyphenols present in VCO may decrease the lipid levels (cholesterol, TAG and phospholipids) compared with other oils. Some of the beneficial metabolic actions of polyphenols are mediated by their ability to activate sirtuin 1 (SIRT1)( Reference Lagouge, Argmann and Gerhart-Hines 50 , Reference Baur, Pearson and Price 51 ) or AMP-activated protein kinase (AMPK)( Reference Zang, Xu and Maitland-Toolan 52 ). SIRT1 is a NAD+-dependent class III histone deacetylase( Reference Haigis and Guarente 53 ). Polyphenols stimulate hepatic SIRT1 activity and reduce lipid accumulation( Reference Zang, Xu and Maitland-Toolan 52 ); however, the underlying mechanisms remain unclear. There are reports that the polyphenols substantially prevent the impairment in phosphorylation of AMPK and its downstream target, ACC, elevation in the expression of FAS and lipid accumulation in human HepG2 hepatocytes( Reference Hou, Xu and Maitland-Toolan 54 ).
Furthermore, increased rates of mitochondrial and peroxisomal β-oxidation were observed in VCO-fed rats, which was evident from the increased activities of CPT I and ACO in VCO-fed rats compared with the other oil-fed rats. The OO-enriched diet showed a maximum decrease in the activity of CPT I, resulting in increased hepatic lipid accumulation in OO-fed rats. The present results are in agreement with previous reports( Reference Ferramosca, Savy and Zara 55 ). Moreover, increased activities of mitochondrial β-oxidation enzymes, namely ACDH, enoyl CoA hydratase, β-hydroxy acyl CoA dehydrogenase and 3-ketoacyl CoA thiolase, were observed in VCO-fed rats compared with the rats fed the other oils. There are reports that laurate present in coconut oil has a higher oxidation rate than oleate( Reference Piot, Hocquette and Veerkamp 56 ) and the metabolites derived from long-chain fatty acids decrease activity of CPT I and the efficiency of ATP production( Reference Hocquette and Bacchart 57 ). In addition, medium-chain TAG in coconut oil are transported predominantly in portal venous blood, extensively oxidised in the liver and less effectively incorporated into tissue lipids than other long-chain fatty acids( Reference Scheig, Senior, Van Itallie and Greenberger 58 ). Thus lauric acid present in VCO may be partly responsible for the increased catabolism of fats and decreased accumulation of lipids.
In addition, an increased rate of peroxisomal β-oxidation was observed in VCO-fed rats, which was evident from the increased activity of ACO in peroxisomes compared with other oil-fed rats. There are reports that long-chain fatty acids (>C20) require peroxisomal β-oxidation to shorten the chain length for further completion of oxidation in mitochondria. Thus both the substrate specificities and alterations in the activities of the β-oxidation enzymes may account for the lipid-lowering effects of dietary VCO. It has been reported that polyphenols can also increase peroxisomal β-oxidation and can suppress body fat accumulation( Reference Fukuchi, Hiramitsu and Okada 49 ); thus, higher amounts of polyphenols in VCO may beneficially regulate fatty acid oxidation. The present study for the first time has evidence that dietary VCO increases the activities of both mitochondrial and peroxisomal enzymes involved in the fatty acid oxidation pathway.
The role of transcriptional regulation of fatty acid metabolism has been studied extensively. Reports indicate that dietary fatty acids suppress hepatic lipogenesis by reducing the transcription of genes coding for the participating enzymes( Reference Jump, Thelen and Mater 59 , Reference Clarke 60 ). Several lines of evidences have shown that down-regulation of SREBP-1c is a primary mechanism by which dietary fatty acids reduce hepatic lipogenesis. SREBP-1c stimulates the transcription of the enzymes that participate in the synthesis of fatty acids and TAG( Reference Horton, Goldstein and Brown 8 ); there was a down-regulation in the hepatic mRNA expression of FAS and its transcription factor SREBP-1c in VCO-fed rats compared with other oils. SREBP-1c is known to be regulated by the nutritional status of the individual and there are reports that polyphenols can reduce SREBP-1c expression( Reference Mukai, Sun and Sato 61 , Reference Murase, Misawa and Minegishi 62 ). Since VCO contains higher amounts of polyphenols( Reference Nevin and Rajamohan 17 , Reference Arunima and Rajamohan 20 ), this may down-regulate the hepatic expression of SREBP-1c and hence reduce lipogenesis. A potential link between SIRT1 and SREBP-1c has also been suggested( Reference Rodgers and Puigserver 63 ). Considerable evidence suggests that SIRT1 functions as a master metabolic regulator by either directly modifying histones or by indirectly regulating the activities of several transcriptional regulators( Reference Rodgers and Puigserver 63 , Reference Rodgers, Lerin and Haas 64 ). There are reports that polyphenols increase SIRT1 deacetylase activity( Reference Hou, Xu and Maitland-Toolan 54 ), deacetylation of SREBP-1c by SIRT1 inhibits SREBP-1c activity by decreasing its stability and its association with its lipogenic target gene promoters( Reference Rodgers and Puigserver 63 , Reference Ponugoti, Kim and Xiao 65 ).
Moreover, both mitochondrial and peroxisomal β-oxidation systems are under the transcriptional regulation of PPARα, which is the nuclear receptor activated by natural ligands such as fatty acids( Reference Reddy and Hashimoto 10 ). There are reports that medium-chain fatty acids are the natural PPAR ligands( Reference Marcelo, Alessandro and Steven 66 ) and supplementation of coconut oil up-regulates the mRNA expression of PPARα( Reference Bonilla, Redonnet and Noël-Suberville 67 ). Moreover, polyphenols are reported to be the natural PPARα activators( Reference Fukuchi, Hiramitsu and Okada 49 , Reference Kaul, Sikand and Shukla 68 ). PPARα activation up-regulates fatty acid oxidation-related genes such as ACO, CPT I, etc.( Reference Pineda Torra, Gervois and Staels 69 ) and suppresses postprandial lipidaemia and lipid accumulation through the enhancement of fatty acid oxidation( Reference Kimura, Takahashi and Murota 70 ). An up-regulation in the mRNA expression of PPARα and its target genes involved in fatty acid oxidation was observed in VCO-fed rats compared with the other oil-fed rats; these results were correlated with the increased activities of enzymes involved in mitochondrial and peroxisomal β-oxidation in VCO-fed rats. Thus, from the present results it is clear that supplementation of VCO regulates fatty acid oxidation partly via PPARα-dependent pathways.
In conclusion, dietary VCO beneficially modulates lipid levels and fatty acid metabolism by transcriptional regulation of the enzymes involved in the synthesis and oxidation of fatty acids.
Acknowledgements
Financial assistance in the form of a research fellowship from the University of Kerala (grant number 5825/2009) to S. A. is gratefully acknowledged; the University of Kerala had no role in the design, analysis or writing of this article.
S. A. designed and performed the experiment, analysed the data and prepared the manuscript. T. R. was responsible for the very concept and design of the study, interpretation of the data and formulation of research questions. All authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.












