Inflammatory bowel disease (IBD), which includes Crohn's disease and ulcerative colitis, refers to chronic inflammatory disorders that may affect the entire gastrointestinal tract( Reference Kaser, Zeissig and Blumberg 1 ). The precise aetiology of IBD remains unidentified( Reference Maloy and Powrie 2 ). However, several factors including genetic predisposition, host immune system and intestinal microenvironment have been proposed to make major contributions to the pathogenesis of this disease( Reference Kaser, Zeissig and Blumberg 1 ). Intestinal homeostasis depends on the dynamic crosstalk among the microbiota, intestinal epithelium and local immune cells( Reference Maloy and Powrie 2 ). Dysregulation or breakdown of the interactions between them may precipitate the intestinal inflammation observed in IBD.
The intestinal epithelium is lined by a single layer of epithelial cells that forms a physical barrier and is crucial for intestinal homeostasis. Small-intestinal intraepithelial lymphocytes (IEL) are a unique population of T cells that reside in the paracellular space between the epithelial cells. IEL serve as the first line of immune defence against invading pathogens and preserve the integrity of the mucosal barrier( Reference Cheroutre, Lambolez and Mucida 3 ). Gut IEL include two phenotypically distinct populations: conventional IEL, which express T-cell receptor (TCR)αβ and either co-receptor CD4 or CD8αβ heterodimers, and unconventional IEL, which express either TCRγδ or TCRαβ and bear CD8αα+ co-receptor molecules( Reference Konkel, Maruyama and Carpenter 4 ). Small-intestinal IEL include a large proportion of γδ-T cells with a predominant CD4−CD8+ phenotype, and most of them express a homodimeric form of the CD8αα+ co-receptor molecule( Reference Pardigon, Darche and Kelsall 5 ). γδ-IEL help preserve the integrity of a damaged epithelial surface by facilitating the localised delivery of an epithelial cell growth factor( Reference Chen, Chou and Fuchs 6 ). The depletion of colonic γδ-T cells aggravates dextran sodium sulphate (DSS)-induced colitis. The protective effect of γδ-T cells has been confirmed in various IBD animal models( Reference Kuhl, Pawlowski and Grollich 7 ).
Glutamine (Gln) is the most abundant free amino acid in the mammalian plasma and intracellular pool. Gln has various physiological functions. It is essential for the proliferative response of enterocytes and rapidly dividing immune cells( Reference Wilmore and Shabert 8 ). Numerous studies have shown that Gln has immunomodulatory properties. Previous studies have found that Gln supplementation enhances stress protein responses, attenuates inflammatory reactions, improves tissue metabolic functions and reduces oxidative stress under catabolic conditions( Reference Wischmeyer 9 ). A study carried out by Nose et al. ( Reference Nose, Yang and Sun 10 ) has shown that Gln prevents total parenteral nutrition-associated changes in the phenotype and function of small-intestinal IEL and preserves the function of the epithelial barrier. Our previous study has also revealed that Gln administration prevents the apoptosis of small-intestinal IEL γδ-T cells and down-regulates γδ-T-cell-expressed inflammatory mediators in septic mice( Reference Lee, Hu and Ko 11 ). The DSS colitis murine model is usually used to assess novel treatments for IBD( Reference Egger, Bajaj-Elliott and MacDonald 12 ). DSS-induced damage is not restricted to the colon; there are also morphological and biochemical changes throughout the small-intestinal mucosa( Reference Yazbeck, Howarth and Butler 13 ). To date, no study has investigated the effects of Gln on small-intestinal IEL γδ-T-cell subset distribution and subsequent inflammatory mediator changes in IBD. We hypothesised that Gln increases the proportion of cells of the TCRγδ subset of small-intestinal IEL and down-regulates the expressions of inflammation-associated mediators, which may attenuate DSS-induced small-intestinal mucosal injury. Therefore, in the present study, we examined the expressions of genes involved in inflammatory responses and in the maintenance of small-intestinal epithelial tissue integrity in DSS-treated mice.
Materials and methods
Animals
Male C57BL/6 mice aged 8–12 weeks and weighing 22–25 g at the beginning of the experiment were used in the present study. All the mice were housed in cages in a temperature- and humidity-controlled room and were allowed free access to a standard chow diet for 1 week before the start of the experiment. The mice were cared for in full compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996), and protocols were approved by the Institutional Animal Care and Use Committee of Taipei Medical University.
Experimental procedures
A total of twenty-four mice were assigned to one normal control (NC) group and two DSS-treated groups, with each group having eight mice. The NC group and one of the DSS-treated groups (DSS-C) were fed a common semi-purified diet, while the other DSS-treated group (DSS-G) was fed an identical diet, except that part of casein was replaced by Gln, which provided 25 % of total amino acid nitrogen (Table 1). The amount of Gln used is known to have an immunomodulatory effect on catabolic conditions( Reference Yeh, Hsu and Yeh 14 ). The diets were fed for 10 d during the experimental period. Mice in the NC group were given distilled water, whereas the DSS-treated groups were given distilled water containing 2·5 % (w/v) DSS (molecular weight 40 kDa; MP Biomedicals) during the last 5 d (on days 6–10) of the experiment. During the experimental period, the body weight of the mice was recorded daily. At the end of the experiment (day 11), the mice were anaesthetised using an intraperitoneal Zoletil (20 mg/kg) injection and killed by cardiac puncture. To obtain peritoneal lavage fluid, 2 ml of sterile saline were injected into the peritoneal cavity of the mice. Peritoneal lavage fluid samples were stored at − 80°C until inflammatory cytokine analysis. Small-intestinal tissue samples were collected for a histological examination. The small-intestinal IEL γδ-T-cell subset was prepared for gene expression analysis.
Table 1 Composition of the experimental diets (g/kg)

* The salt mixture contained the following (mg/g): calcium phosphate dibasic, 500; NaCl, 74; potassium sulphate, 52; potassium citrate monohydrate, 20; magnesium oxide, 24; manganese carbonate, 3·5; ferric citrate, 6; zinc carbonate, 1·6; cupric carbonate, 0·3; potassium iodate, 0·01; sodium selenite, 0·01; chromium potassium sulphate, 0·55.
† The vitamin mixture contained the following (mg/g): thiamin hydrochloride, 0·6; riboflavin, 0·6; pyridoxine hydrochloride, 0·7; nicotinic acid, 3; calcium pantothenate, 1·6; d-biotin, 0·05; cyanocobalamin, 0·001; retinyl palmitate, 1·6; dl-α-tocopherol acetate, 20; cholecalciferol, 0·25; menaquinone, 0·005.
Measurements of the concentrations of inflammatory cytokines in peritoneal lavage fluid
The concentrations of IL-1β, TNF-α, IL-6 and macrophage chemoattractant-1 in peritoneal lavage fluid were measured using ELISA kits according to the manufacturer's instructions (eBioscience).
Preparation of small-intestinal intraepithelial lymphocytes
Small-intestinal IEL were isolated as described previously, with some modifications( Reference Chennupati, Worbs and Liu 15 ). After the removal of Peyer's patches and mesenteric fat tissue, the small intestine was cut longitudinally, and the contents were flushed out with cold Ca–Mg-free PBS (Ca2+, Mg2+-free PBS with 1 mm-HEPES, 2·5 mm-NaHCO3 and 2 % fetal bovine serum at pH 7·3). Later, the small intestine was cut into 5 mm pieces. Tissue pieces were placed in 20 ml of Roswell Park Memorial Institute (RPMI) 1640 medium with 10 % fetal bovine serum and incubated for 30 min at 37°C with gentle shaking. The tissue pieces and medium were transferred into a 50 ml centrifuge tube and vortexed at the maximum setting for 15 s, and the supernatant was discarded. This step was repeated twice using fresh medium each time, and cells in the supernatants from each treatment were pooled. The supernatants were pooled and filtered through a 40 μm nylon mesh, pelleted at 1600 rpm for 10 min and resuspended in 40 % Percoll (GE Healthcare) in RPMI 1640 medium. This cell suspension was overlaid with 70 % Percoll in RPMI 1640 medium and centrifuged at room temperature and 2800 rpm for 20 min without interruption. Small-intestinal IEL were recovered from the 40 %/70 % interphase and washed twice with RPMI 1640 medium.
Flow cytometric analysis
CD103 is an integrin expressed by IEL. CD103 mediates the adhesion of lymphocytes to epithelial cells by interacting with their epithelium-specific ligands. To determine the percentages of γδ-T cells+ belonging to the small-intestinal IEL population, the co-expression of CD103+ and CD8αα+ by γδ-T cells was measured. A total of one million small-intestinal IEL were suspended in 100 μl of staining buffer. IEL were incubated with purified rat anti-mouse CD16/CD32 (2.4G2; BD Bioscience) at 4°C for 10 min followed by staining with Pacific Blue anti-mouse CD103 (2E7; Biolegend), allophycocyanin anti-mouse TCRγ/δ (GL3; Biolegend), phycoerythrin anti-mouse CD8α (53-6.7; Biolegend) and fluorescein isothiocyanate anti-mouse CD8β (YTS156.7.7; Biolegend) at 4°C for 15 min. After two washes, the cells were studied using a BD FACSCantoII flow cytometer (BD Bioscience).
Isolation of total small-intestinal intraepithelial lymphocyte γδ-T cells
To study gene expressions in γδ-T cells, total small-intestinal IEL were stained with allophycocyanin anti-mouse TCRγ/δ (GL3; Biolegend), phycoerythrin anti-mouse CD8α (53-6.7; Biolegend) and fluorescein isothiocyanate anti-mouse CD8β (YTS156.7.7; Biolegend). Small-intestinal IEL were purified on a cell sorter (BD FACSAria III; BD Biosciences). The TCRδ+/CD8β− subsets were isolated from γδ-T cells, and the purity ( ≥ 98 %) was checked.
mRNA extraction and quantitative real-time RT PCR analysis
Total RNA was isolated from the purified small-intestinal γδ-IEL using the RNeasy Mini Kit (QIAGEN). The RNA pellet was dissolved in RNase-free water. The total RNA solution was stored at − 80°C for subsequent assays. The concentration of RNA was determined and quantified by measuring absorbance at 260 and 280 nm on a spectrophotometer. Complementary DNA was prepared from total RNA using the RevertAid™ First Strand cDNA Synthesis Kit (Fermentas) according to standard protocols. RT was carried out by subsequent incubation for 5 min at 65°C, 60 min at 42°C and 5 min at 70°C. Complementary DNA was stored at − 80°C until use. Specific mRNA genes were amplified by real-time RT-PCR using the 7300 Real-Time PCR System (Applied Biosystems) with SYBR Green I as the detection format. The primers of the housekeeping gene (β-actin), TNF-α, interferon-γ (IFN-γ), IL-4, IL-17, complement 5a receptor (C5aR), keratinocyte growth factor (KGF) and regenerating islet-derived protein III-γ (RegIIIγ) were purchased from Mission Biotech based on the deposited complementary DNA sequences (GenBank database, NCBI). The sequences of primers used for the quantitative RT-PCR assays are listed in Table 2. Amplification was carried out in a total volume of 25 μl containing 1 × Power SYBR Green PCR Master Mix (Applied Biosystems), 400 nm of each primer and 100 ng of complementary DNA. The reaction was carried out using one cycle of 2 min at 50°C and 10 min at 95°C, followed by forty cycles of 15 s at 95°C and 1 min at 60°C, with a final dissociation curve analysis. Expression levels were quantified in duplicate by means of a real-time RT-PCR. Cycle threshold (C t) values for the genes of interest were normalised to that of mouse β-actin and were used to calculate the relative mRNA expression levels.
Table 2 Sequences of primers used for inflammatory mediator quantitative real-time PCR assays
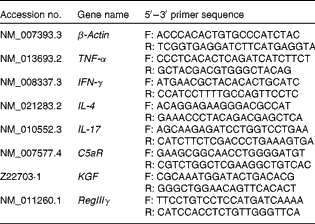
F, forward primer; R, reverse primer; IFN-γ, interferon-γ; C5aR, C5a receptor; KGF, keratinocyte growth factor; RegIIIγ, regenerating islet-derived protein III-γ.
Histopathological and immunohistological examinations
The middle segments of small-intestinal tissues were collected and fixed with 4 % buffered paraformaldehyde overnight. The small-intestinal tissue samples were embedded in paraffin blocks (Leica Instruments). The small-intestinal tissue blocks were sectioned into 5 μm-thick sections and mounted on glass slides. To detect the integrity of tight junctions (TJ), TJ protein ZO-1 immunohistochemical staining was carried out. The tissue sections were deparaffinised and rehydrated with xylene and graded alcohol. After the retrieval of antigen with 10 mm-citrate buffer (pH 6·0), the sections were incubated with a primary antibody against ZO-1 (1:300; Abcam) overnight at 4°C, followed by incubation with a biotinylated secondary antibody (dilution 1:300; Chemicon) for 1 h at room temperature. After carrying out the reaction with the peroxidase-linked avidin–biotin complex (Vector) for 1 h at room temperature, a diaminobenzidine solution kit (Vector) was used to detect ZO-1 immunoreactivity. Haematoxylin (Sigma) nuclear staining was carried out to contrast-stain cell nuclei and cytoplasm. Digital images at 100 × and 200 × magnifications per section were captured with a Zeiss Axiophot light microscope equipped with a digital camera (Carl Zeiss). All the tissue sections were covered with cover slips using Permount (Fisher Scientific) and measured using the digital image analysis system (Image Pro Plus 5.1, Media Cybernetics). The ‘count/size’ and ‘area’ commands were used to determine the intensity of ZO-1 immunoreactivity. The automatic object counting and measuring processes were used to quantify the immunoreactive areas in the sections. Values are expressed as μm2. At least eight microscopic fields per section and three independent samples for each group were analysed, and averaged areas were obtained for each group.
Statistical analysis
All the data are expressed as means and standard deviations. Differences among the groups were analysed by a one-way ANOVA using Tukey's multiple-comparison test. A P value < 0·05 was considered to be statistically significant.
Results
Percentages of CD103+/T-cell receptor γδ+/CD8αα+ T cells in small-intestinal intraepithelial lymphocytes
Compared with the NC group, the DSS-treated groups had lower percentages of small-intestinal IEL CD103+/TCRγδ+/CD8αα+ T cells. The percentages of CD103+/TCRγδ+/CD8αα+ T cells belonging to the small-intestinal IEL population were significantly higher in the DSS-G group than in the DSS-C group at the end of the experiment (Fig. 1).
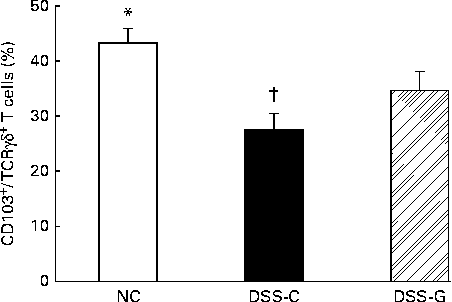
Fig. 1 Distributions of γδ-T cells in small-intestinal intraepithelial lymphocytes in the normal control (NC) group, dextran sulphate sodium (DSS)-treated group fed the control diet (DSS-C) and DSS-treated group fed the glutamine diet (DSS-G) for 10 d and given DSS water for 5 d. All the data are representative of duplicate measurements (n 6). Values are means, with standard deviations represented by vertical bars. Differences among the groups were analysed by a one-way ANOVA with Tukey's multiple-comparison test. * Mean value was significantly different from those of the other two groups (P <0·05). † Mean value was significantly different from that of the DSS-G group (P <0·05).
Concentrations of cytokines in peritoneal lavage fluid
The concentrations of IL-1β, IL-6, TNF-α and macrophage chemoattractant-1 were significantly higher in the peritoneal lavage fluid of the DSS-C group than in that of the other two groups. The DSS-G group had lower concentrations of cytokines than the DSS-C group, and it exhibited no difference when compared with the NC group (Table 3).
Table 3 IL-1, IL-6, TNF-α and macrophage chemoattractant protein-1 (MCP-1) concentrations in peritoneal lavage fluid† (Mean values and standard deviations)

NC, normal control group; DSS-C, dextran sulphate sodium (DSS)-treated group fed the control diet; DSS-G, DSS-treated group fed the glutamine diet.
* Mean values were significantly different from those of the other two groups (P <0·05).
† All the data are representative of duplicate measurements (n 8). Differences among the groups were analysed by a one-way ANOVA with Tukey's multiple-comparison test.
Expressions of proinflammatory mediator mRNA in small-intestinal γδ-intraepithelial lymphocytes
The expression levels of TNF-α, IFN-γ, IL-17 and C5aR genes were higher in the DSS-C group than in the NC and DSS-G groups. There were no differences in the expression levels of these genes between the NC and DSS-G groups (Fig. 2).
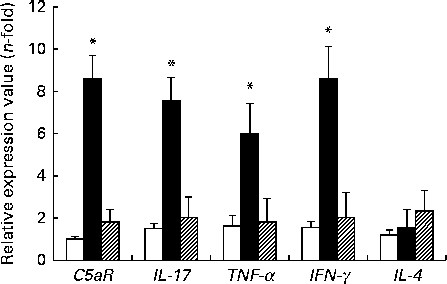
Fig. 2 Expressions of C5a receptor (C5aR), IL-17, TNF-α, interferon-γ (IFNγ) and IL-4 mRNA in small-intestinal intraepithelial lymphocyte γδ-T cells. All the data are representative of duplicate measurements (n 6). Values are means, with standard deviations represented by vertical bars. Differences among the groups were analysed by a one-way ANOVA with Tukey's multiple-comparison test. □, Normal control group; ■, dextran sulphate sodium (DSS)-treated group fed the control diet; ![]() , DSS-treated group fed the glutamine diet. *Mean values were significantly different from those of the other two groups (P <0·05).
, DSS-treated group fed the glutamine diet. *Mean values were significantly different from those of the other two groups (P <0·05).
Expressions of keratinocyte growth factor-1 and regenerating islet-derived protein III-γ genes in small-intestinal γδ-intraepithelial lymphocytes
The expression of KGF-1 mRNA was significantly higher in the DSS-C group than in the NC and DSS-G groups. There were no differences in the expression of RegIIIγ gene between the NC and DSS-treated groups (Fig. 3).
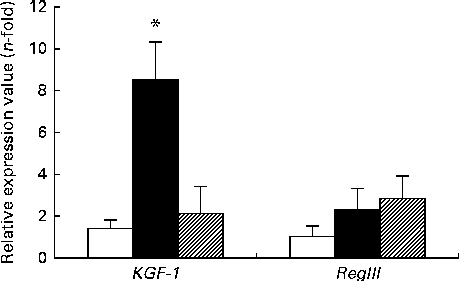
Fig. 3 Expressions of regenerating islet-derived protein III-γ (RegIIIγ) and keratinocyte growth factor-1 (KGF-1) mRNA in small-intestinal intraepithelial lymphocyte γδ-T cells. All the data are representative of duplicate measurements (n 6). Values are means, with standard deviations represented by vertical bars. Differences among the groups were analysed by a one-way ANOVA with Tukey's multiple-comparison test. □, Normal control group; ■, dextran sulphate sodium (DSS)-treated group fed the control diet; ![]() , DSS-treated group fed the glutamine diet. * Mean value was significantly different from those of the other two groups (P <0·05).
, DSS-treated group fed the glutamine diet. * Mean value was significantly different from those of the other two groups (P <0·05).
Histopathological aspects and tight junction protein ZO-1 distribution in the small-intestinal mucosa
The expression of ZO-1 in the small-intestinal mucosa was examined by immunohistochemistry, which revealed that the epithelial structure differed among the distinct groups. In the NC group, the epithelium of the small-intestinal mucosa was intact. The small-intestinal mucosa of the DSS-C group exhibited villus denudation, subepithelial space formation and epithelial lifting. A less extensive ZO-1 distribution was also observed in the DSS-C group. The extent of small-intestinal epithelial damage was less severe and the expression of ZO-1 was more obvious in the DSS-G group than in the DSS-C group. The representative images obtained for each group are shown in Fig. 4(a) (left: 100 × magnification of images; right: 200 × magnification of the images). The quantification of ZO-1-immunoreactive areas among the groups is shown in Fig. 4(b). The immunoreactive areas of the DSS-G and NC groups were significantly larger than those of the DSS-C group. No differences in ZO-1-immunoreactive areas were found between the NC and DSS-G groups.

Fig. 4 Distribution of tight junction protein ZO-1-immunoreactive areas in the small-intestinal mucosa of mice in the normal control (NC) group, dextran sulphate sodium (DSS)-treated group fed the control diet group (DSS-C) and DSS-treated group fed the glutamine diet (DSS-G). (a) Representative histological images of mice in the normal control and DSS-treated groups at 100 × (left column) and 200 × (right column) magnifications. Cell nuclei were contrast-stained with haematoxylin. Arrows indicate ZO-1-positive areas. The normal small intestine exhibited intact epithelium with marked dark-brown ZO-1 expression. In contrast, mucosal structural degeneration and less extensive ZO-1 distribution were observed in the DSS-C group. It is obvious that changes in the DSS-C group were more severe than those in the DSS-G group. (b) Quantification of ZO-1-immunoreactive areas among the groups. The immunoreactive areas of the DSS-G and NC groups were significantly larger than those of the DSS-C group. *Value was significantly different from those of the other two groups (P <0·05). (A colour version of this figure can be found online at http://journals.cambridge.org/bjn).
Discussion
DSS is a sulphated polysaccharide that is commonly used to induce colitis in rodent models. The damage induced by DSS is believed to result from acute chemical toxicity within the colonic epithelium. A recent study has indicated that DSS-induced damage extends to the small intestine by changes in histopathology and an increase in neutrophil activity. Whether the effects of DSS in the small intestine result from a DSS-mediated effect or a compensatory response secondary to colonic changes remains unclear( Reference Yazbeck, Howarth and Butler 13 ). In the present study, we did observe a lower percentage of CD103/TCRγδ+/CD8αα+ T cells belonging to the small-intestinal IEL population after DSS administration and found that Gln administration increased the proportion of small-intestinal IEL γδ-T cells in the DSS-treated mice.
Murine γδ-T cells comprise 5–10 % of the total T-cell population. γδ-IEL are involved in intestinal immunoregulation and serve as the first line of defence against infectious agents( Reference Kuhl, Pawlowski and Grollich 7 ). Intestinal γδ-T cells produce a variety of cytokines including IFN-γ, IL-4 and IL-17 upon bacterial infection( Reference Cheng, Cui and Shao 16 , Reference Inagaki-Ohara, Sakamoto and Dohi 17 ). IL-17 is a cytokine that plays an important role in the orchestration of innate immune function. IL-17 induces pro- and anti-inflammatory responses. Although Ogawa et al. ( Reference Ogawa, Andoh and Araki 18 ) have reported that the blockade of IL-17 function by injection of IL-17 antibodies intraperitoneally aggravates DSS-induced colitis, the overexpression of IL-17 has been found to increase neutrophil infiltration, which may result in chronic inflammation and tissue damage( Reference Park, Li and Yang 19 ). Previous studies have shown that the expression of IL-17 mRNA is up-regulated in inflamed mucosa. The serum concentrations of IL-17 have also been reported to be elevated in IBD patients( Reference Fujino, Andoh and Bamba 20 , Reference Seiderer, Elben and Diegelmann 21 ). Complement C5a is an important proinflammatory mediator and can activate innate immune cells( Reference Peng, Li and Wang 22 ). In addition, C5a has been shown to play an important role in the regulation of the function of γδ-T cells( Reference Han, Geng and Li 23 ). C5a directly acts on the C5aR expressed by γδ-T cells, resulting in the activation of cells, and subsequently enhances their capacity to produce IL-17( Reference Cheng, Cui and Shao 16 ). Our findings of elevated concentrations of C5aR accompanied by an increased expression of IL-17 in the DSS-C group indicated that small-intestinal IEL γδ-T cells were activated and an inflammatory reaction was initiated in response to DSS. These findings were consistent with the higher concentrations of TNF-α and IFN-γ expressed by small-intestinal IEL γδ-T cells in the DSS-C group. TNF-α is an important mediator involved in the onset and regulation of inflammatory and immune responses( Reference Beutler and Cerami 24 ). A previous study has found that IL-17-producing T cells also secrete IFN-γ( Reference Annunziato, Cosmi and Santarlasci 25 ). In a study carried out by Tajima et al. ( Reference Tajima, Wakita and Noguchi 26 ), naïve CD8+ cells were transferred into recombination activating gene (RAG)-deficient mice. In that study, it was found that mice with severe colitis had IL-17- and IFN-γ-double-positive cells in mesenteric lymph nodes. Alternatively, the transfer of naïve CD8+ T cells derived from either IL-17- or IFN-γ-knockout mice was found to be associated with less severe colitis, indicating that IL-17 and IFN-γ can cooperate to cause pathology in this model of colitis. Kuhl et al. ( Reference Kuhl, Pawlowski and Grollich 7 ) also showed that early intestinal γδ-T-cell depletion resulted in the increased production of IFN-γ by lamina propria lymphocytes and splenocytes, which may consequently aggravate intestinal inflammation.
KGF, a member of the fibroblast growth factor family, promotes the growth of intestinal epithelial cells. Activated γδ-IEL release KGF near damaged epithelial cells and help maintain and restore the integrity of epithelial tissues after injury( Reference Chen and Kaiser 27 ). A previous study has shown that KGF-knockout mice are more susceptible to DSS( Reference Kuhl, Pawlowski and Grollich 7 ). RegIIIγ is a bactericidal lectin secreted into the bowel lumen. A previous study has found that the in vivo depletion of RegIIIγ from the small intestine decreases bacterial death, whereas mice with recombinant RegIIIγ have been found to exhibit enhanced small-intestinal bacterial clearance( Reference Brandl, Plitas and Schnabl 28 ). A study carried out by Okamoto & Sasaki( Reference Okamoto and Sasaki 29 ) has found that the expression of RegIII mRNA is enhanced in gut epithelial cells of DSS-treated animals and IBD patients. The findings of the present study indicated that the expression of KGF by small-intestinal γδ-T cells was enhanced at the mRNA level in the DSS-treated mice, suggesting that repair mechanisms had been activated. That the expression of RegIIIγ gene did not differ among the groups suggests that bactericidal activity was not involved in the present experimental conditions.
In the present study, we found that Gln administration had some effects on the responses of small-intestinal IEL γδ-T cells, which were not observed in the DSS-treated mice without Gln supplementation. First, Gln administration reversed the decrease in the percentage of small-intestinal IEL γδ-T cells induced by DSS. Our previous study has also shown that Gln supplementation increases the percentage of small-intestinal IEL γδ-T cells in a septic condition( Reference Lee, Hu and Ko 11 ). Second, the concentrations of small-intestinal IEL γδ-T-cell-expressed inflammatory mediators, including C5aR, IL-17, TNF-α and IFN-γ, and inflammatory cytokines secreted into the abdomen were lower in the DSS-G group. These results indicate that small-intestinal inflammation was less severe when Gln was administered. Our findings are consistent with a previous report, which has also found that pretreatment with Gln prevents increases in the expressions of blood IFN-γ and IL-17 in acute DSS-induced colitis( Reference Chu, Hou and Pai 30 ). To better understand the intercellular barrier between epithelial cells, a protein of TJ was studied. TJ form an apical barrier to the paracellular movement of water, solutes and immune cells in polarised epithelium( Reference Fanning, Jameson and Jesaitis 31 ). ZO-1 is a membrane phosphoprotein expressed by TJ of both epithelial and endothelial cells( Reference Willott, Balda and Fanning 32 ). Histological findings of the present study revealed a higher expression of ZO-1 in the DSS-G group, indicating that the extent of small-intestinal mucosal damage was less severe when Gln was administered. Since the severity of small-intestinal epithelial injury was ameliorated, the expression of the repair gene, KGF, may thus have been lowered, as observed in the DSS-G group. A previous study carried out by Nose et al. ( Reference Nose, Yang and Sun 10 ) has demonstrated that Gln administration in mice receiving total parenteral nutrition partially prevents the expression of small-intestinal IEL-derived cytokines, enhances the expression of TJ proteins, and leads to a significant improvement in the function of small-intestinal epithelial barrier.
The beneficial effects of Gln with regard to the attenuation of DSS-induced small-intestinal epithelial injury may involve a complex mechanism. Changes in the expression levels of small-intestinal IEL γδ-T-cell-expressed inflammatory mediators may be only one of several contributory mechanisms. A previous study has found that Gln prevents the activation of NF-κB and enhances the expression of organ heat shock proteins, thus attenuating organ injury in catabolic conditions( Reference Singleton and Wischmeyer 33 ). In addition, the ability of Gln to reduce oxidative stress may also play a role in the elevation of the expressions of inflammatory mediators. Glutathione is a major antioxidant and acts as a vital component in host defence. Gln has been found to be rate limiting for glutathione synthesis, and the availability of Gln is critical for the generation of glutathione stores( Reference Welbourne 34 ). However, the mechanism responsible for the protective effect of Gln on small-intestinal epithelial injury requires further investigation.
In summary, the present study has shown for the first time that Gln administration enhances the proportion of γδ-T cells and down-regulates the expression of inflammation-associated mediator genes by small-intestinal IEL γδ-T cells. Histological findings revealed that damage to the small-intestinal mucosa was less severe in the DSS-G group. This result indicates that pretreatment with Gln suppresses the expression of T helper type 1/T helper type 17-associated cytokines by small-intestinal IEL γδ-T cells and may consequently reduce the small-intestinal inflammatory responses, thus ameliorating the severity of DSS-induced small-intestinal epithelial injury.
Acknowledgements
The present study was supported by a research grant (NSC99-2320-B-034-002-MY3) from the National Science Council, Taipei, Taiwan. The National Science Council had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: M.-H. P. and C.-L. Y. contributed to the concept of the study and carried out most of the data analysis; J.-J. L. carried out part of the analysis; S.-L. Y. and W.-J. C. helped interpret the data; C.-L. Y. and S.-L. Y. prepared the manuscript.
None of the authors has any conflicts of interest.









