There is evidence that the likelihood of experiencing stressful life events (SLEs) is partly under genetic control (Reference McGuffin, Katz and AldrichMcGuffin et al, 1988a ; Reference Plomin, Lichtenstein and PedersenPlomin et al, 1990; Kendler et al, Reference Kendler, Neale and Kessler1993a , Reference Kendler, Kessler and Walters1995). As SLEs play an important role in provoking a depressive episode (Reference Paykel, Myers and DieneltPaykel et al, 1969; Reference Bebbington, Tennant and HurryBebbington et al, 1981; Reference Brown, Harris, Brown and HarrisBrown & Harris, 1989; Reference Mackinnon, Henderson and AndrewsMackinnon et al, 1990; Reference Dohrenwend, Shrout, Link and MazureDohrenwend et al, 1995 Reference KesslerKessler, 1997), part of the familial clustering seen in depression may be due to the genetically mediated tendency of some individuals to experience more depressogenic life events than others. Evidence suggests that around 10-15% of genetic effects on liability for depression may in fact be mediated by a mechanism whereby individuals select themselves into high-risk environments (Reference Kendler and Karkowski ShumanKendler & Karkowski Shuman, 1997). This mechanism is referred to as geneenvironment correlation or genetic control of exposure to the environment (Reference Kendler and EavesKendler & Eaves, 1986; Reference Susser, Susser and SusserSusser & Susser, 1987; Reference OttmanOttman, 1990; Reference Rutter, Dunn and PlominRutter et al, 1997). Any genetic effects on predisposition to experience SLEs are likely to be mediated by heritable personal characteristics (Reference Champion, Goodall and RutterChampion et al, 1995; Reference Thapar and McGuffinThaper & McGuffin, 1996; Reference Saudino, Pedersen and LichtensteinSaudino et al, 1997). The underlying mechanism of gene-environment correlation would thus be one of person-environment correlation. The personality trait neuroticism (N), or negative affectivity, is a plausible candidate because: (a) it is a strong predictor of onset of depression (Reference ParkerParker, 1980; Reference Clayton, Ernst and AngstClayton et al, 1989; Reference Fergusson, Horwood and LawtonFergusson et al, 1989; Reference Andrews, Stewart and Morris-YatesAndrews et al, 1990; Rodgers, 1990b; Reference Boyce, Parker and BarnettBoyce et al, 1991; (b) several studies have suggested that N is an independent predictor of exposure to life events (Reference Nelson and CohenNelson & Cohen, 1983; Reference Horwood and FergussonHorwood & Fergusson, 1986; Reference Fergusson and HorwoodFergusson & Horwood, 1987; Reference Aldwin, Levenson and SpiroAldwin et al, 1989; Reference Heady and WearingHeady & Wearing, 1989; Reference Magnus, Diener and FujitaMagnus et al, 1993); and (c) it is subject to genetic effects which are shared to a large degree with depression (Reference Eaves and EysenckEaves & Eysenck, 1976; Reference Pedersen, Plomin and McClearnPedersen et al, 1988; Reference LoehlinLoehlin, 1992; Reference Kendler, Neale and KesslerKendler et al, 1993b ; Reference Eaves, Heath and NealeEaves et al, 1998).
Similarly, cognitive ability is another possible factor mediating person-environment relationships. Lower childhood cognitive ability (CA) has been identified as an independent developmental risk factor for both childhood and adult-onset affective and neurotic disorder in two separate birth cohort studies (Reference Crow, Done, Sacker, Hafner and GattazCrow et al, 1995; Reference Van Os, Jones and LewisVan Os et al, 1997). It has been suggested that individual differences in cognitive ability have a role in the experience of and subsequent coping with life events (Reference Masten, Garmezy and TellegenMasten et al, 1988; Reference McNally and ShinMcNally & Shin, 1995; Reference Cowen, Wyman and WorkCowen et al, 1996; Reference Tiet, Bird and DaviesTiet et al, 1998). Lower cognitive competence may result in higher rates of SLEs through reduced ability to cope with potentially threatening events (Reference KesslerKessler, 1997).
The main methodological problem in studies examining the relationship between N and CA on the one hand, and SLEs in relation to mental health outcomes on the other, is that the direction and independence of any effect is difficult to establish, because exposures and outcomes are generally assessed at around the same time. For example, life events may cause depression, which in turn can influence the level of N through both ‘scar’ and ‘state’ effects (Reference Zeiss and LewinsohnZeiss & Lewinsohn, 1988), creating a spurious association between N and SLEs. This can be prevented by ensuring that the trait N is assessed prior to any depressive state. Similarly a spurious association between N and SLEs may be found because high N increases the risk of depression, which in turn can lead to increased experience of SLEs (Reference KesslerKessler, 1997). Allowing for the association between N, CA and mental health, and the bidirectional association between mental health and SLEs, is therefore necessary in uncovering any independent relationship between N and CA on the one hand, and SLEs on the other.
The study described here used a 43-year follow-up of a general population cohort of 5362 individuals to further examine the relationships between N and CA assessed during childhood, and adult life events and depression. In a previous study with this sample (Reference Van Os and JonesVan Os & Jones, 1999), evidence was found that both N and CA influenced the occurrence of life events. This study was conducted to assess in more detail the paths leading from personality characteristics to occurrence of life events, using a path-analytic approach (Reference WrightWright, 1934). The hypotheses were: (a) that survey members with higher N and lower CA in childhood would be more likely to report stressful life events, independent of mental health outcome; and (b) that the association between personality trait and SLEs was specific for neuroticism, thus would not be evident for the personality trait extraversion.
METHOD
Sample
The Medical Research Council National Survey of Health and Development (NSHD) is an ongoing longitudinal study of a stratified sample of 5362 participants (males 52.5%), born in Britain during the week 3-9 March 1946.
Data were collected on eleven occasions at regular intervals until age 16 years, and on nine occasions thereafter, the most recent contact occurring in 1989 when the subjects were aged 43 years (Reference Atkins, Cherry, Douglas, Mednick and BaertsAtkins et al, 1981; Reference WadsworthWadsworth, 1991).
Neuroticism
Survey members had completed the six neuroticism (N) and the six extraversion (E) items of the short Maudsley Personality Inventory (MPI) at the age of 16 years - each item contributing up to 2 points, range 0-12 for each scale (Reference EysenckEysenck, 1958).
Childhood cognitive ability
Childhood CA was measured by non-verbal, verbal and reading ability tests administered at ages 8, 11 and 15 years, vocabulary at ages 8 and 11 years, and arithmetic tests at ages 11 and 15 years (Reference Pidgeon and DouglasPidgeon, 1964). Test scores of survey members had been ‘normalised’, so that the population mean was 100 and the standard deviation 15 (Reference Andrews, Morgan and SonquistAndrews et al, 1973). At each age, principal component analysis of these highly correlated educational test variables yielded a general measure of CA, explaining around 75% of the variance. The principal components of the cognitive test scores at ages 8, 11 and 15 years were strongly correlated; the principal component at age 15 years, which was found to be the strongest cognitive predictor of adult mental health in earlier studies (Reference Jones, Rodgers and MurrayJones et al, 1994; Reference Van Os, Jones and LewisVan Os et al, 1997), was used in the analyses. Missing values at age 15 years were imputed if the principal components of cognitive test scores at ages 8 and/or 11 years were available.
Life events
Stressful life events were recorded using a face-to-face structured interview by trained nurses at subject ages 36 and 43 years. At age 36 years, event information was collected on eight events, whereas at age 43 years a more comprehensive list of 17 items was administered. Events at both ages were rated retrospectively for the period of the preceding 12 months.
Events included reported deaths (of relatives or friends), accidents, injuries, moving house, illness, divorce or separation, burglaries and robberies, worries and crises arising from work, family, children and spousal discord. In 1982, most items were about events happening to others (friends, family), whereas in 1989 items were roughly equally divided into events to others and events to the person interviewed. For each event it was recorded whether it had occurred (score=1) or not (score=0). At both ages 36 and 43 years, a continuous aggregated life-event score was constructed by adding the scores for each event. Means and standard deviations were similar for corresponding scores at ages 36 and 43 years. Events were also scored according to emotional impact (0, no event; 1, event that left the individual fairly calm; 2, event that left the individual shocked but able to cope; 3, event that left the individual rather overwhelmed), and life change (age 36 years: 0, no event; 1, event occurred but has not changed life in any way; 2, event occurred and has changed life; age 43 years: 0, no event; 1, event occurred but way of life not at all changed; 2, somewhat changed; 3, changed a great deal). The total scores of both emotional impact and life change showed near-perfect correlations with the aggregated life-event score (Spearman's rho r=0.93 and r=0.95, respectively, P<0.001).
Adult mental health
When survey participants were 36 years old, a short version of the Present State Examination (PSE) was administered by trained lay interviewers (Rodgers & Mann, 1986), including all PSE neurotic and affective features. At age 43 years, the Psychiatric Symptom Frequency (PSF) scale (Rodgers, 1996) was administered through a structured interview by trained nurses. The PSF scale is a 19-item scale rating symptoms of anxiety and depression for the 12 months preceding the interview. The reliability, validity and internal consistency of PSE and PSF in this sample were found to be satisfactory (Rodgers & Mann, 1986; Rodgers, 1996; Reference Lindelow, Hardy and RodgersLindelow et al, 1997). Total symptom scores were calculated for both PSE and PSF.
Analyses
Path analysis allows combination of several related regression equations into a single model (Reference WrightWright, 1934). We compared, using the M-plus program (Reference Muthen and MuthenMuthen & Muthen, 1998), the goodness of fit of four related models which were nested in each other (Fig. 1). As it has been suggested that the most satisfactory approach to assessing the fit of a model may be by some form of cross-validation procedure (Reference Everitt, Dunn, Everitt and DunnEveritt & Dunn, 1991), the result obtained with the measures of SLEs and mental health at age 43 years were used to validate the results obtained with the same measures at age 36 years. In the first model (hereafter ‘full model’), a (bidirectional) correlational relationship was assumed between SLEs and mental health. In addition, the influence of N at age 16 years and CA at age 15 years on (a) later mental health and (b) experience of SLEs was modelled. The specificity of any influence of N on adult SLEs was examined by also allowing for an influence of childhood E on adult experience of SLEs. The second model differed from the first in that the parameter of the association between N and SLEs was constrained to zero (hereafter ‘N-constrained model’). In the third model, the parameter between CA and SLEs was constrained to zero (hereafter ‘CA-constrained model’). Finally, in the fourth model, the parameter of the association between E and SLEs was constrained to zero (hereafter ‘E-constrained model’). If our initial hypotheses were correct, the fit of the first model should be better than the second and the third, whereas no difference should be apparent between the first and the fourth model. The fit of the model was assessed using the maximum likelihood method and chi-squared test statistic.

Fig. 1 Path analytic model.
The modelling procedure started with the full model as depicted in Fig. 1. The fit of the model was satisfactory at both age 36 years and age 43 years (respectively: χ2=3.9, d.f.=2, P=0.14 and χ2=1.2, d.f.=2, P=0.53). Further improvement of fit of the full model was obtained by removing redundant paths (i.e. paths representing weak and non-significant associations in the model).
Risk set
Of the original 5362 survey members, 3322 were successfully interviewed in 1982 at age 36 years, and 3262 in 1989 at age 43 years. The 1982 risk set consisted of individuals with complete data on childhood N, childhood E, 1982 PSE symptoms and 1982 life events (n=2481). The 1989 risk set consisted of individuals with complete data on childhood N, childhood E, 1989 PSF symptoms and 1989 life events (n=1757).
RESULTS
Sample and models
The number of men in the risk set was 1247 (50%) at age 36 years, and 931 (53%) at age 43 years. Mean mental health, SLE and N scores are set out in Table 1. At both ages, the full model provided a much better fit to the data than the N-constrained model (Tables 2 and 3). No such differences were apparent in the comparisons between the full model and the E-constrained model (Tables 2 and 3). The full model was also better than the CA-constrained model, but this was only apparent at age 36 years, and the effect of CA was such that higher CA predicted more SLEs (Table 2). Comparisons of the models stratified by gender did not show large differences between men and women (age 36 years: comparison full model and N-constrained model, men: χ2=22.3, d.f.=1, P<0.001; women: χ2=16.3, d.f.=1, P<0.001; comparison full model and CA-constrained model, men: χ2=11.0, d.f.=1, P<0.001; women: χ2=16.1, d.f.=1, P<0.001; age 43 years: comparison full model and N-constrained model, men: χ2=13.1, d.f.=1, P<0.001; women: χ2=3.56, d.f.=1, P=0.059).
Table 1 Sample characteristics

| Age 36 years (n=2481) | Range | Age 43 years (n=1757) | Range | |
|---|---|---|---|---|
| Mean neuroticism score, age 16 years (s.d.) | 6.05 (3.66) | 0 to 12 | 5.98 (3.62) | 0 to 12 |
| Mean extraversion score, age 16 years (s.d.) | 7.91 (2.76) | 0 to 12 | 8.04 (2.72) | 0 to 12 |
| Mean PSE (age 36 years) or PSF (age 43 years) score (s.d.) | 2.31 (3.89) | 0 to 38 | 10.30 (10.25) | 0 to 92 |
| Mean life-event score (s.d.) | 1.97 (1.46) | 0 to 7 | 1.60 (1.42) | 0 to 9 |
| Mean cognitive ability score (s.d.) | 0.09 (0.89) | -2.7 to 2.7 | 0.13 (0.85) | -3.0 to 2.7 |
Table 2 Comparison between full and constrained models at age 36 years (n=2481); paths as in Fig. 1

| Path | Coefficient | Standard error | P | |
|---|---|---|---|---|
| Cognitive ability→life events | 0.17 | 0.032 | <0.001 | |
| Cognitive ability→mental health | NS1 | |||
| Extraversion→life events | NS1 | |||
| Full model | Extraversion→mental health | -0.131 | 0.027 | <0.001 |
| Neuroticism→life events | 0.052 | 0.008 | <0.001 | |
| Neuroticism→mental health | 0.22 | 0.021 | <0.001 | |
| Life events⇌mental health | 0.92 | 0.11 | <0.001 | |
| Extraversion→neuroticism | -1.06 | 0.20 | <0.001 | |
| Full model fit | χ2=5.72, d.f.=4, P=0.22 | |||
| Difference N-constrained model2 | χ2=42.50, d.f.=1, P<0.001 | |||
| Difference E-constrained model3 | Not applicable as path extraversion→life events not significant | |||
| Difference CA-constrained model4 | χ2=26.98, d.f.=1, P<0.001 | |||
Table 3 Comparison between full and constrained models at age 43 years (n=1757); paths as in Fig. 1
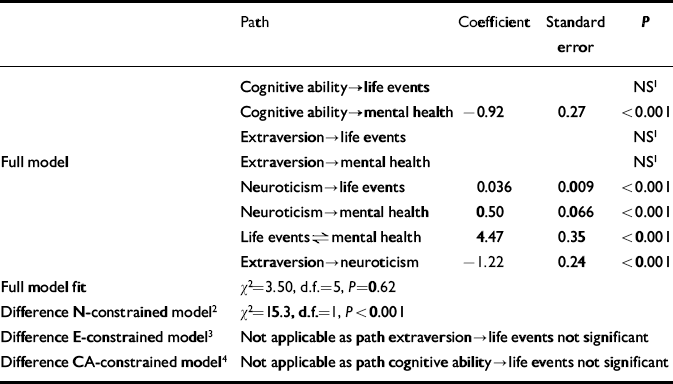
| Path | Coefficient | Standard error | P | |
|---|---|---|---|---|
| Cognitive ability→life events | NS1 | |||
| Cognitive ability→mental health | -0.92 | 0.27 | <0.001 | |
| Extraversion→life events | NS1 | |||
| Full model | Extraversion→mental health | NS1 | ||
| Neuroticism→life events | 0.036 | 0.009 | <0.001 | |
| Neuroticism→mental health | 0.50 | 0.066 | <0.001 | |
| Life events⇌mental health | 4.47 | 0.35 | <0.001 | |
| Extraversion→neuroticism | -1.22 | 0.24 | <0.001 | |
| Full model fit | χ2=3.50, d.f.=5, P=0.62 | |||
| Difference N-constrained model2 | χ2=15.3, d.f.=1, P<0.001 | |||
| Difference E-constrained model3 | Not applicable as path extraversion→life events not significant | |||
| Difference CA-constrained model4 | Not applicable as path cognitive ability→life events not significant |
Missing data
The above findings could be biased, if, for example, differential attrition existed for individuals with low levels of N but high rates of SLEs, or high levels of N and low rates of SLEs. In order to examine the possibility of such a bias, frequencies of SLEs were compared in those with and without missing data on N, E and CA, and levels of N, E and CA were compared in those with and without missing SLEs at either age 36 or age 43 years. These comparisons showed no differential effect of attrition for any of the variables, with the exception of significant differences in the level of CA as a function of missing SLEs at both age 36 and 43 years, and in the level of E at age 43 years (Table 4).
Table 4 Mean values of neuroticism (N), extraversion (E), cognitive ability (CA) and stressful life event (SLE) frequency as a function of attrition

| Subjects with: | Mean N (s.d.) | n | Mean E (s.d.) | n | Mean CA (s.d.) | n | Mean SLE age 36 (s.d.) | n | Mean SLE age 43 (s.d.) | n |
|---|---|---|---|---|---|---|---|---|---|---|
| Missing N age 16 years | - | - | - | - | - | - | 1.95(1.44) | 539 | 1.67(1.52) | 395 |
| Non-missing N age 16 years | - | - | - | - | - | - | 1.95(1.45) | 2633 | 1.60(1.42) | 1904 |
| Missing E age 16 years | - | - | - | - | - | - | 1.92(1.42) | 573 | 1.64(1.51) | 419 |
| Non-missing E age 16 years | - | - | - | - | - | - | 1.96(1.46) | 2599 | 1.60(1.42) | 1880 |
| Missing CA age 15 years | - | - | - | - | - | - | 1.98(1.38) | 214 | 1.60(1.56) | 167 |
| Non-missing CA age 15 years | - | - | - | - | - | - | 1.95(1.45) | 2958 | 1.61(1.43) | 2132 |
| Missing SLEs at age 36 years | 5.96(3.71) | 1170 | 7.83(2.74) | 1161 | -0.084(0.96)1 | 1497 | - | - | - | - |
| Non-missing SLEs at age 36 years | 6.05(3.67) | 2633 | 7.90(2.76) | 2599 | 0.034(0.91)1 | 2958 | - | - | - | - |
| Missing SLEs at age 43 years | 6.02(3.73) | 1899 | 7.73(2.78)1 | 1880 | -0.083(0.98)1 | 2323 | - | - | - | - |
| Non-missing SLEs at age 43 years | 6.02(3.63) | 1904 | 8.03(2.72)1 | 1880 | 0.078(0.87)1 | 2132 | - | - | - | - |
DISCUSSION
The result showed that higher levels of N measured at age 16 years increased the likelihood of experiencing SLEs in adulthood, independent of the reciprocal association between SLEs and mental health.
The pattern of results was similar for men and women.
There were several, inevitable, layers of attrition in the data. First, of the original 5362 survey members, ‘only’ 3322 were successfully interviewed in 1982 at age 36 years, and 3262 in 1989 at age 43 years. Previous examinations have shown that any bias due to selective drop-out was slight (Reference Atkins, Cherry, Douglas, Mednick and BaertsAtkins et al, 1981; Rodgers, 1990a; Reference Wadsworth, Mann and RodgersWadsworth et al, 1992). Second, in order to stabilise the sample, a number of individuals with missing data were excluded. Although there may have been some systematic difference between the original sample of 5362 individuals and the groups of 2481 and 1757 subjects used in the current analyses, testing for systematic bias that could have led to spurious results suggested this was unlikely. Although there were statistically significant differences in the level of E and the level of CA as a function of missing SLEs (Table 4), the absolute size of the difference was very small, and level of SLEs did not differ as a function of missing values of E and CA.
The separate analyses at ages 36 and 43 years do not, of course, constitute a true cross-validation of the findings, as the childhood exposures were the same and repeated measures in the same individuals were used rather than measures collected in two independent samples. Nevertheless, the temporal stability of the association between N and SLEs enhances the validity of the hypothesised effect.
Mental health was defined continuously for the purposes of this investigation. Continuous measures avoid loss of information through arbitrary dichotomisation, and may constitute a more valid indicator of non-psychotic mental health problems than diagnostic categories (Reference Rose and BarkerRose & Barker, 1978; Reference Lewis and WesselyLewis & Wessely, 1990; Reference Anderson, Huppert and RoseAnderson et al, 1993; Reference GoldbergGoldberg, 1996), which recent research suggests are diagnostic conventions imposed on a continuum (Reference Kendler and GardnerKendler & Gardner, 1998). Previous work in this sample has shown the validity of the use of continuous mental health scores as the psychiatric outcome in regression analyses (Rodgers, 1990a).
General population surveys have to rely on simple measures. Stressful life events were assessed using a structured interview, which is inferior to detailed semi-structured interviews such as the Life Events and Difficulties Schedule (LEDS) (Reference Brown, Harris, Brown and HarrisBrown & Harris, 1989) and does not allow clear separation into personal and network events, or avoidable and unavoidable events. We used a continuous life-event score rather than a dichotomous life-event exposure. The fact that our life-event score was nearly perfectly correlated with total scores of life-event emotional impact and life change validates the use of a continuous score, because the higher the life-event score, the greater the emotional impact and associated life change.
As mental health was assessed in a prevalence sample, both first-onset and chronic cases were included. It is possible that some individuals with chronic symptoms had higher levels of SLEs because of the complications arising from chronicity itself. Similarly, depressed individuals may have a biased view of events, making them more likely to report an SLE. In the model, this possibility was allowed for by assuming SLEs and mental health were correlated, rather than assuming a unidirectional influence of SLEs on mental health.
Contrary to our initial hypothesis, we found that individuals with higher CA reported more changes to their lives as a result of life events. However, this was found in the data collected at age 36 years, and no such effect was apparent at age 43 years. If it were, nevertheless, a true path association, it could be explained by the fact that associations between cognitive characteristics and reported SLEs may involve effects both at the level of SLEs and at the level of observable exposure to SLEs. Thus, the level of CA may reflect differential appraisal and recall processes rather than differences in the rate of observable external incidents (Reference Rabbitt and McInnisRabbitt & McInnis, 1988). The fact that individuals with higher CA reported more life events may reflect greater awareness of the ramifications and complications following a life event, rather than an observably greater number of SLEs.
Although the effect of neuroticism on SLEs was highly significant, the actual effect size was small. The regression coefficient of the association of N with SLE at age 36 years represented 0.04 SLE standard deviation, and 0.025 SLE standard deviation at age 43 years per 1 point increase in N. Thus, the effect size comparing individuals scoring highest on N (12 points) with those scoring lowest (0 point) was 0.48 s.d. and 0.30 s.d. at ages 36 and 43 years, respectively. Although these latter effect sizes are generally considered respectable (Reference CohenCohen, 1977), they only represent a comparison between extremes. Therefore, according to the data in this investigation the final impact of N on mental health through the mechanism of N-driven SLE exposure is small. The measures used, however, were crude and are likely to have generated enough random error to reduce the true effect size substantially.
CONCLUSION
Survey participants' N, but not E, affected the probability of reported SLE exposure in adult life, independent of mental health. Thus, the previously reported association between N and life-event exposure does not appear to be merely the result of the independent association between N and mental disorder (Reference Nelson and CohenNelson & Cohen, 1983; Reference Fergusson and HorwoodFergusson & Horwood, 1987; Reference Aldwin, Levenson and SpiroAldwin et al, 1989; Reference Heady and WearingHeady & Wearing, 1989; Reference Magnus, Diener and FujitaMagnus et al, 1993). Part of the association between N and mental health may thus be the result of N-driven exposure to depressogenic SLEs. As a substantial part of the familial clustering observed with N can be explained by the effect of shared genes (Reference Eaves and EysenckEaves & Eysenck, 1976; Reference Pedersen, Plomin and McClearnPedersen et al, 1988; Reference LoehlinLoehlin, 1992; Reference Eaves, Heath and NealeEaves et al, 1998), the effect of N on SLEs may in turn explain part of the familial resemblance observed for SLEs (Reference McGuffin, Katz and BebbingtonMcGuffin et al, 1988b ; Reference Plomin, Lichtenstein and PedersenPlomin et al, 1990; Reference Kendler, Neale and KesslerKendler et al, 1993a ; Reference Billig, Hershberger and IaconoBillig et al, 1996; Reference Foley, Neale and KendlerFoley et al, 1996; Reference Thapar and McGuffinThaper & McGuffin, 1996; Reference Saudino, Pedersen and LichtensteinSaudino et al, 1997). Similarly, the suggestion that genetic risk factors for depressive disorder - which are to a large extent shared with genetic risk for N (Reference Kendler, Neale and KesslerKendler et al, 1993b ) - increase the probability of exposure to depressogenic SLEs (Reference Kendler and Karkowski ShumanKendler & Karkowski Shuman, 1997) may be mediated by the effect of neuroticism on SLE exposure. Of course, we do not suggest that the association between N and SLE exposure, and the subsequent onset of psychiatric symptoms, is entirely genetic: it is the impact of the environment (the SLE) that determines the depressogenic effect.
Our sample was not genetically sensitive, and non-genetic developmental interactive processes may contribute to the observed association. The focus of this study, however, was on the possible mechanisms of the previously observed genetic contribution to environmental measures such as SLEs. Our data are compatible with the suggestion that personality measures are likely candidates for such genotype-environment correlations (Reference PlominPlomin, 1994).








eLetters
No eLetters have been published for this article.